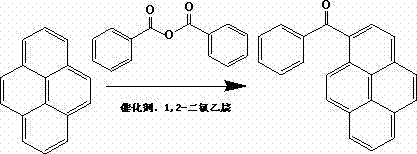
One-step synthesis method of 1-benzoylpyrene
A technology of benzoylpyrene and a synthesis method, which is applied in chemical instruments and methods, condensation preparation of carbonyl compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of difficult recovery of heteropolyacids, equipment corrosion, reaction Short time and other problems, to achieve the effect of good reusability, reducing the pollution of three wastes, and suitable reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0025] Implementation Case 1 Preparation of titania support
[0026] Ti(SO 4 ) 2 solution and NaOH solution, and then the prepared NaOH solution was added dropwise to the Ti(SO 4 ) 2 solution, continue to stir for 2-4 h after the dropwise addition, let it stand overnight, and centrifuge to remove SO 4 2- ions (with BaCl2 Detection of SO 4 2- Whether the ion is removed), the resulting solution is poured out of the polytetrafluoroethylene reactor, 180 ο C under reaction 24 h, transfer out the liquid in the kettle after the end of the reaction, 80 ο C drying, grinding.
Embodiment example 2
[0027] Implementation Case 2 PPML 12 O 40 Catalyst preparation
[0028] Reference: Chen Min, Fine Chemical Industry, 25(112), 2005, 1245-1248
[0029] Make 10 g of phosphotungstic acid and 0.75 g of aluminum nitrate into an aqueous solution of a certain concentration, and slowly add the aqueous solution of aluminum nitrate to the aqueous solution of phosphotungstic acid while stirring at room temperature. h, at 80 ο C evaporate the water to dryness at 100 ο Dry around C, 300 ο C roasting for 3 h, the dry A1PW was obtained 12 O 40 , the catalyst is placed in a desiccator for later use.
Embodiment example 3
[0030] Implementation Case 3 40 % Silica supported aluminum phosphotungstate (40 % AlPW 12 O 40 / SiO 2 ) Catalyst preparation
[0031] Take a certain amount of nano-silica (purchased from Aladdin Company) and impregnate it with 5% hydrochloric acid for 2-3 days, wash with distilled water, centrifuge to neutrality, dry for several hours under infrared lamps, and finally dry at 500 ο After roasting in a C muffle furnace for 3 h, use 4 g of the AlPW prepared in Case 2 12 O 40 The catalyst was dissolved in 20 mL of distilled water, slowly added dropwise to a beaker containing 10 g of the above-mentioned treated silica, and kept stirring, after the dropwise addition, stirred for 12 h, and then stood still for 3 h, and the excess water was passed through 80 ο Evaporate to dryness at C, then at 100 ο Dry at about C, and finally at 300 ο Calcined at C for 3 h, the prepared AlPW with a loading of 40% 12 O 40 / SiO 2 Catalyst, the catalyst is placed in a desiccator for standby...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com