Ribavirin slow release nasal gel used for treating cold and preparation method of gel
A technology for gel and nasal use, applied in the field of medicine, can solve the problems of affecting drug absorption and curative effect, reducing patient compliance, short residence time, etc., and achieve the goal of increasing bioavailability, improving bioavailability and long residence time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 preparation is used for the treatment of the ribavirin sustained-release nasal gel of cold
[0019] Take 5.0g of chitosan, add 900mL of distilled water and 0.1mL of 0.1mol / L hydrochloric acid solution, stir to make it naturally peptized until completely dissolved; then add 5.0g of sodium benzoate and 5.0g of ribavirin, stir well, add distilled water Adjust the total amount to 1000mL, adjust the pH value to 5.8-6.2 with 1.0mol / L sodium hydroxide solution, stir well, and sterilize and dispense.
[0020] Embodiment 2 uses the ribavirin sustained-release nasal gel prepared in embodiment 1 for the treatment of colds to carry out experiments
[0021] 1. In vitro release performance test:
[0022] 1. Purpose of the test:
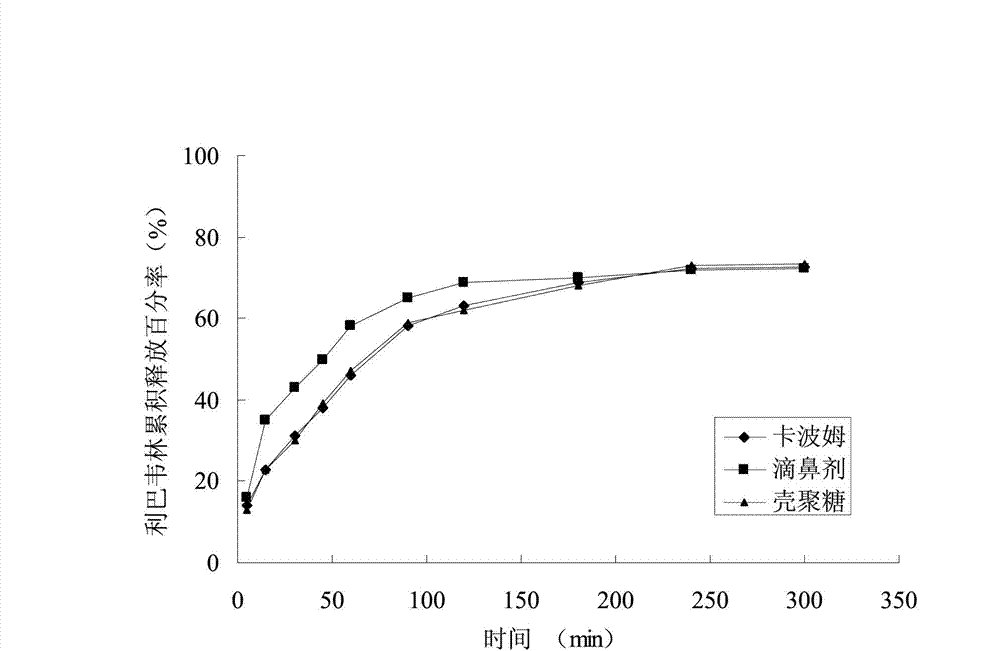
[0023] Observe the sustained-release effect of the ribavirin sustained-release nasal gel prepared in Example 1 for the treatment of colds.
[0024] 2. Test substance:
[0025] The ribavirin slow-release nasal gel (also known as ribavirin nas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com