Kit for synchronously detecting thirty diarrhea pathogens and detection method of kit
A technology for synchronous detection and pathogenic bacteria, applied in the direction of microorganism-based methods, biochemical equipment and methods, and microbial measurement/testing, can solve problems such as low diagnostic efficiency, time-consuming and labor-consuming antibodies, and expensive technical costs. Achieve the effects of saving production cost and detection cost, good sensitivity and specificity, and avoiding low specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
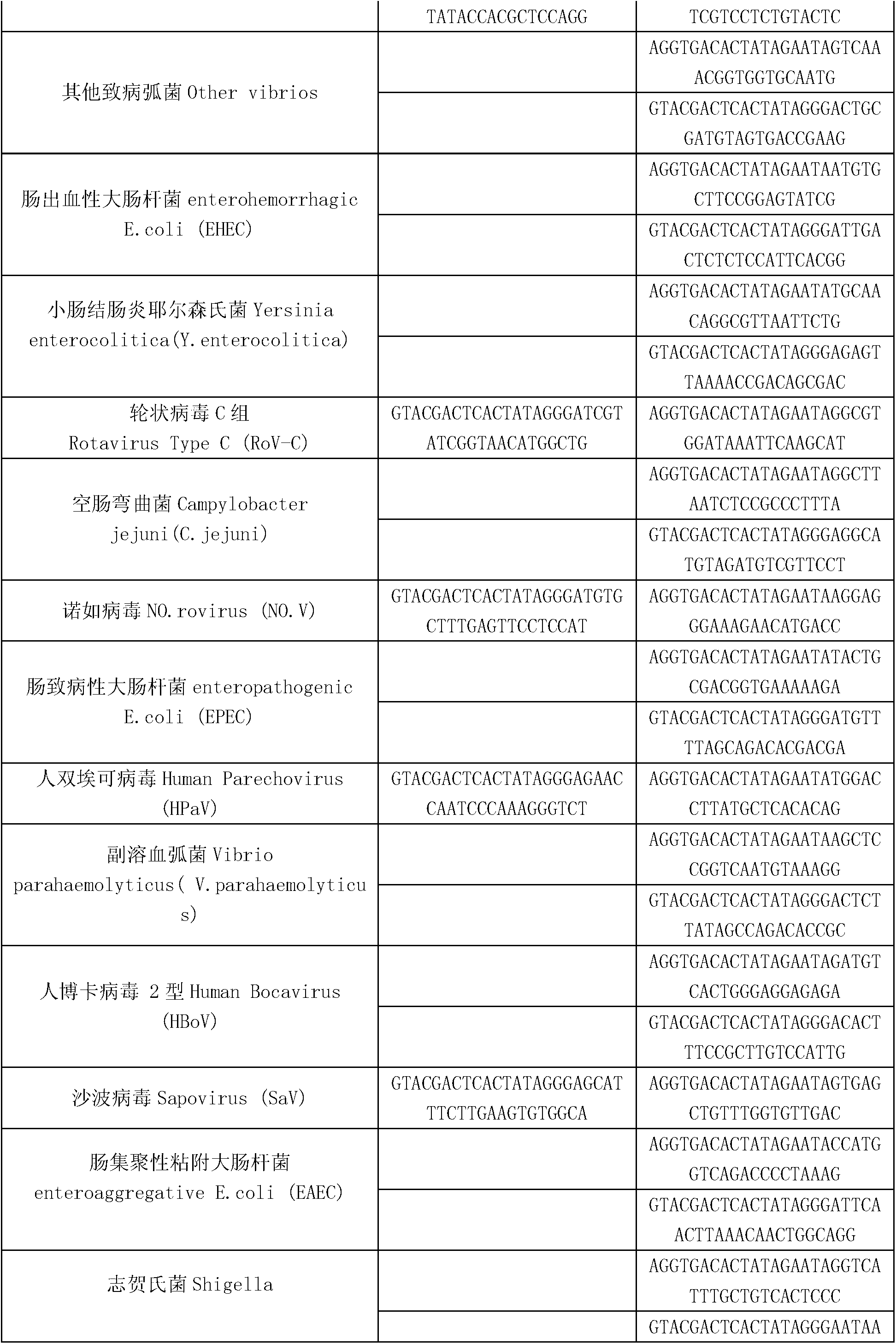
[0037] The present invention is a kit for synchronous detection of thirty kinds of diarrhea pathogenic bacteria, including DEPC water, 5×RT buffer, reverse transcription primer (RT primer mix), RT enzyme (full name reverse transcriptase), X solution, 10×PCR Buffer, PCR Primers, 25mM Magnesium Chloride Solution, DNA Polymerase, Positive Control (DNA Fragment with Specific Sequence, Used for Quality Control of the Whole Reaction System), 5×RT Buffer, 10×PCR Buffer, 25mM Magnesium Chloride Solution and DNA polymerase are ordered from sigma company, and the above-mentioned reverse transcription primers include the RT amplification primers of eleven kinds of diarrhea RNA viruses and human RNA internal reference in Table 1, and the PCR primers include the remaining nineteen kinds of diarrhea-causing viruses in Table 1. Forward and reverse PCR amplification primers of pathogenic bacteria, human DNA internal reference, and reaction internal reference (the upper one is the forward ampli...
specific Embodiment 2
[0079]The present invention is a detection method for synchronous detection of thirty kinds of pathogenic bacteria of diarrhea. The detected pathogens include human enteric coronavirus, enteric adenovirus 40, 41, enterovirus and its subtype (Coxsackievirus A16 type) ), astrovirus, rotavirus groups A, B, C, norovirus, human biechovirus, human bocavirus type 2, sapovirus, small bisnared RNA virus, Shigella, diarrheal coli (EPEC, ETEC, EIEC, EHEC, EAEC), Campylobacter jejuni, Salmonella, pathogenic Vibrio (Vibrio cholerae, Vibrio parahaemolyticus, other pathogenic Vibrio), Yersinia enterocolitica Bacteria, Pseudomonas Shigella-like, Entamoeba histolytica, Giardia, Cryptosporidium, etc. (see Table 1), collect patient samples (feces specimens, vomit, blood, etc.), extract nucleic acid, and take patients The nucleic acid is used as a template for reverse transcription and PCR reactions, and finally the sample is separated by capillary electrophoresis. The specific steps are as follo...
specific Embodiment 3
[0108] Detection Kit Sensitivity and Specificity Analysis
[0109] Sensitivity analysis: After diluting the positive control according to a certain copy number ratio, it is detected by PCR amplification and capillary electrophoresis until no signal is detected. The copy number is the lowest detection line, which is the sensitivity of the kit. The detection sensitivity is up to 40 copies / uL.
[0110] Specificity analysis: Single-plex PCR amplification is detected as a single peak of the target fragment size by capillary electrophoresis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com