Ursolic acid liquid-solid compression tablet
A technology of ursolic acid and compressed tablets, which is applied in the directions of inactive medical preparations, pill delivery, digestive system, etc., can solve problems such as difficulty in utilization, increase difficulty, etc., and achieves low production cost, convenient operation, and disintegration. short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1 prescription:
[0036] The liquid auxiliary material is 1,2-propanediol; the ratio of medicine to liquid is 1:4; the R value is 10; L f The value is 0.300. Each tablet contains ursolic acid 10 mg; 1,2-propanediol 40 mg; microcrystalline cellulose 166.67 mg; micropowder silica gel 16.67 mg; sodium carboxymethyl starch 11.67 mg. Tablet weight is 245.01 mg.
[0037] 2 Preparation method:
[0038] Dissolve and disperse ursolic acid in 1,2-propanediol, gradually add MCC, mix evenly, then add micropowder silica gel, thoroughly pulverize and mix evenly, then add sodium carboxymethyl starch, and directly compress the evenly mixed powder.
Embodiment 2
[0040] 1 prescription:
[0041] The liquid auxiliary material is 1,2-propanediol; the ratio of medicine to liquid is 1:4; the R value is 20; L f The value is 0.225. Each tablet contains ursolic acid 10 mg; 1,2-propanediol 40 mg; microcrystalline cellulose 222.22 mg; micropowder silica gel 11.11 mg; sodium carboxymethyl starch 14.17 mg. Tablet weight is 297.50 mg.
[0042] 2 Preparation method:
[0043] Dissolve and disperse ursolic acid in 1,2-propanediol, gradually add MCC, mix evenly, then add micropowder silica gel, thoroughly pulverize and mix evenly, then add sodium carboxymethyl starch, and directly compress the evenly mixed powder.
Embodiment 3
[0045] 1 prescription:
[0046] The liquid auxiliary material is polyethylene glycol 400; the ratio of medicine to liquid is 1:4; the R value is 10; L f The value is 0.314. Each tablet contains ursolic acid 10 mg; polyethylene glycol 400 40 mg; microcrystalline cellulose 159.24 mg; micropowder silica gel 15.92 mg; sodium carboxymethyl starch 11.26 mg. Tablet weight is 236.42 mg.
[0047] 2 Preparation method:
[0048] Dissolve and disperse ursolic acid in polyethylene glycol 400, gradually add MCC, mix evenly, then add micropowder silica gel, thoroughly pulverize and mix evenly, then add sodium carboxymethyl starch, and directly compress the evenly mixed powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
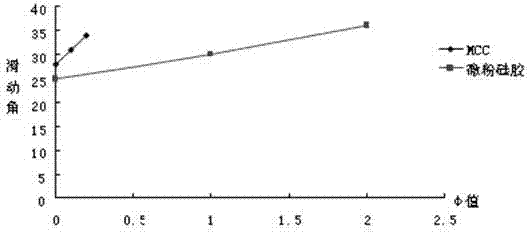
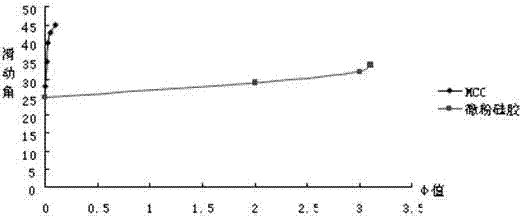
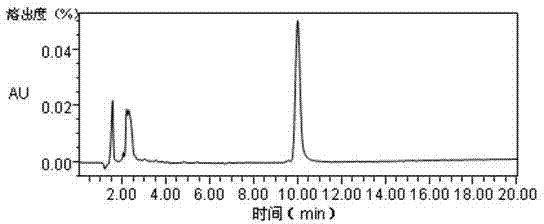
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com