Preparation method of divalent inactivated vaccines for duck virus hepatitis
A technology for duck viral hepatitis and bivalent inactivated vaccine, which can be used in antiviral agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., and can solve problems such as lack of cross-protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
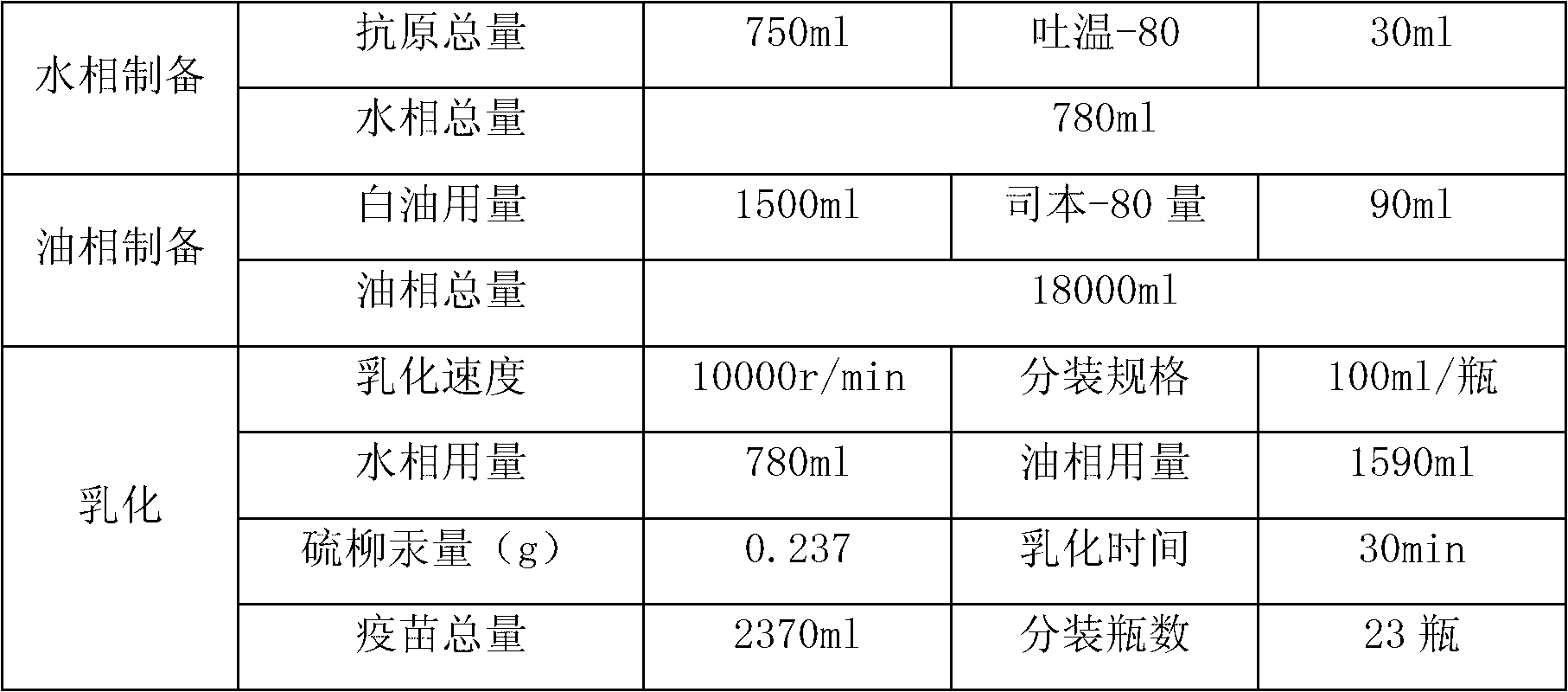
[0047] (1) Vaccine (immunogen) preparation:
[0048] 1 Preparation of virus solution for seedling production
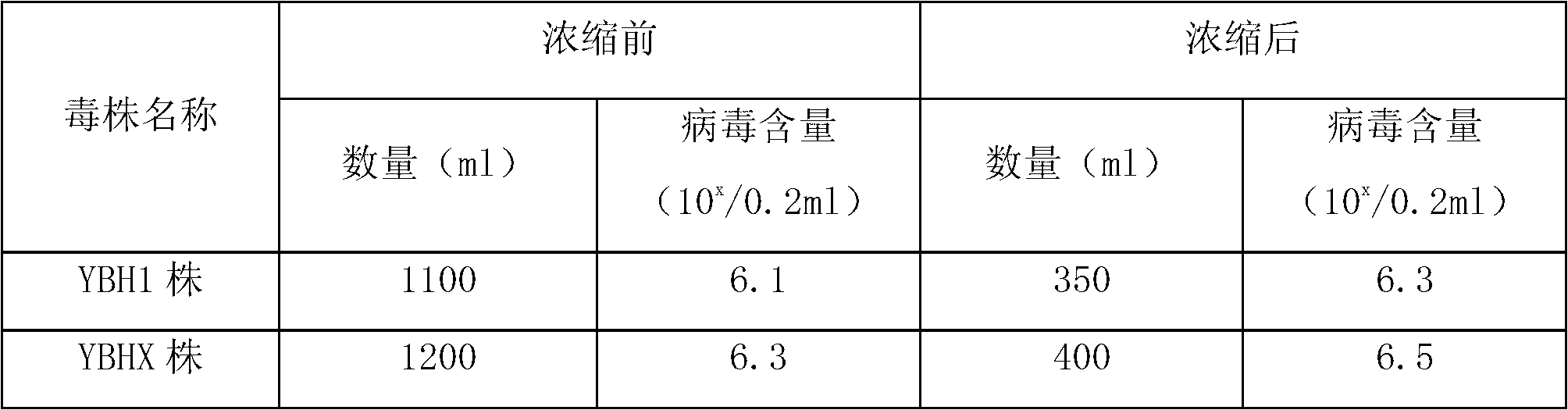
[0049] Preparation of YBH1 strain virus liquid: Dilute the virus strain YBH1 used for production to 100 times, inoculate 100 10-day-old SPF chicken embryos into the allantoic cavity, 0.2ml per embryo, incubate at 36-37°C, and inspect the embryos twice a day. Chicken embryos that died within 48 to 120 hours after inoculation were placed at 2 to 8°C for 4 to 12 hours, and allantoic fluid, amniotic fluid and embryo bodies were collected, and the heads and limbs were removed from the embryo bodies. Homogenize with embryo fluid, freeze and thaw three times, centrifuge at 4000r / min for 30min, take the supernatant and mix it in a sterile container, store at 2-8°C. (See Table 1).
[0050] Preparation of YBHX strain virus liquid Dilute the virus species YBHX strain used for production by 100 times, inoculate 100 susceptible duck embryos of 12-13 days old in the allantoic cav...
Embodiment 2
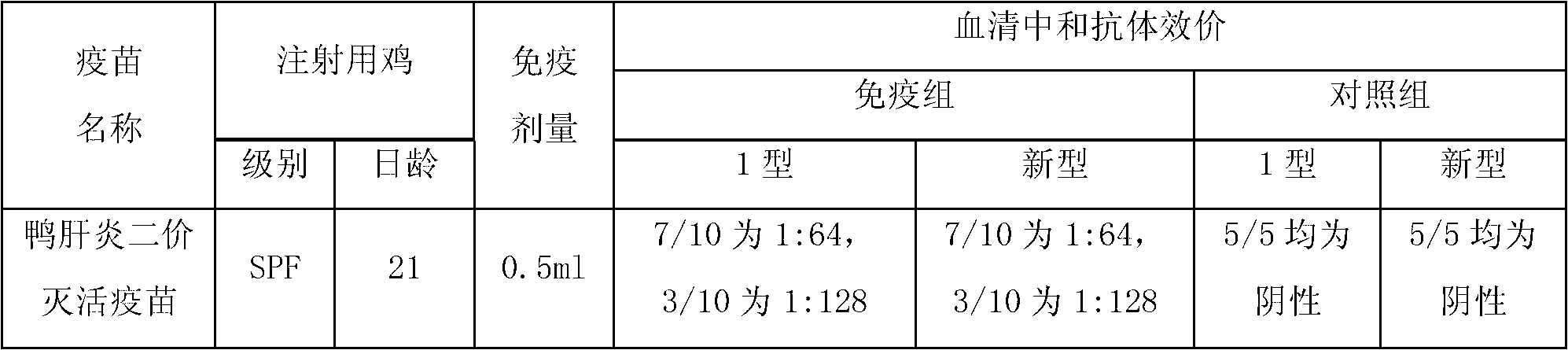
[0071] ——Inspection of finished vaccines
[0072] 1 character
[0073] (1) Appearance Milky white emulsion.
[0074] (2) Dosage form Water-in-oil type, take a clean straw, absorb a small amount of vaccine and drop it in cold water, and it will not spread in the form of oil droplets.
[0075] (3) Stability Take 10ml of the vaccine and put it into a centrifuge tube, centrifuge at 3000r / min for 15 minutes, no water phase will precipitate out at the bottom of the tube.
[0076] (4) Viscosity is 61.6 cp according to the appendix of the current version of "Chinese Veterinary Pharmacopoeia".
[0077] 2 Inspection of the filling volume is carried out according to the appendix of the current version of "Chinese Veterinary Pharmacopoeia", and it meets the standard.
[0078] 3 Sterility test According to the appendix of the current version of "Chinese Veterinary Pharmacopoeia", the sterile growth is carried out.
[0079] 4 For safety inspection, 10 SPF chickens aged 21 to 35 days wer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com