Water-free pharmaceutical compositions suitable for local anaesthetics
A technology for local anesthetics and compositions, applied in the field of anhydrous pharmaceutical compositions, can solve problems such as not mentioned, and achieve the effect of simplifying the sterilization process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1. Methods of Manufacturing and Evaluating Lipid-Based Local Anesthetic-Containing Formulations
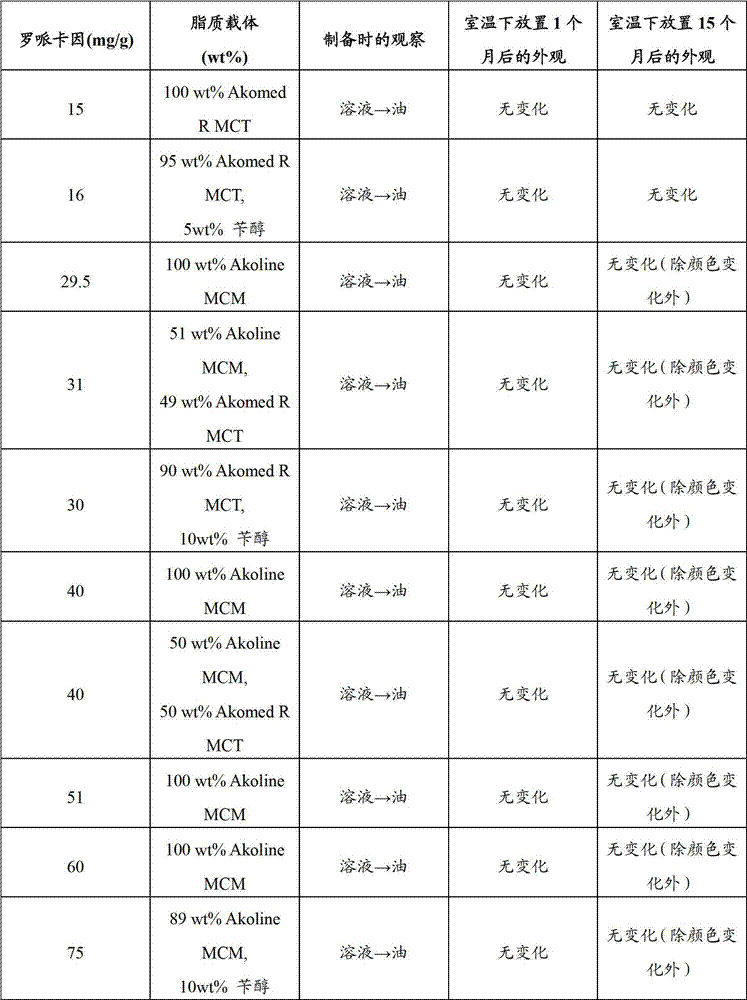
[0035] The following procedure was used to prepare lipid formulations containing ropivacaine and lidocaine for in vitro testing in batch sizes of 20-200 g.
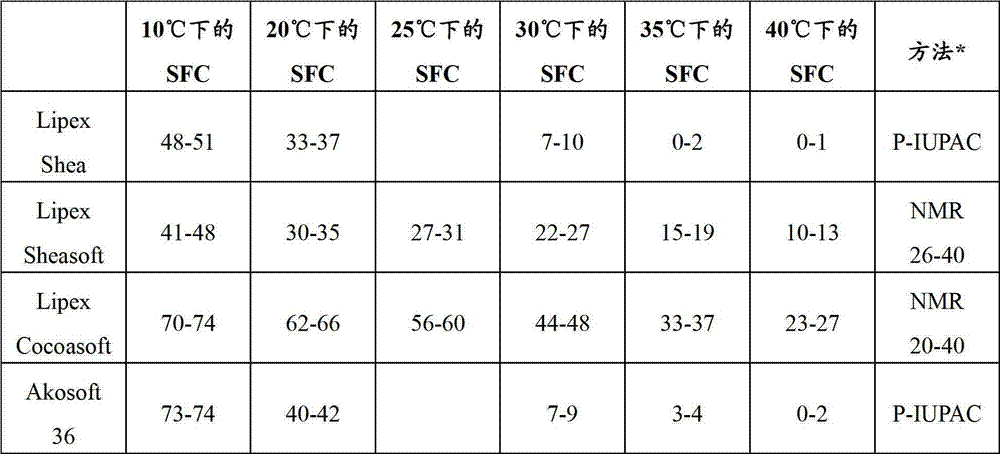
[0036] Weigh out the desired amount of local anesthetic in a 250ml (1000ml) round bottom flask. Absolute ethanol is added in an amount of approximately 20-25 ml for each gram of local anesthetic. The mixture was treated with an ultrasonic water bath set at 50-65° C., depending on the solid fat content of the lipid used, until a clear liquid was obtained. This usually completes within a few minutes. The lipid components (see below) are then added and the resulting mixture is subjected to an ultrasonic water bath until a clear homogeneous liquid is obtained.
[0037] The alcohol was evaporated on a rotary evaporator at a pressure of about 25 mbar and a temperature of about 40-60° C. until the weight of the f...
Embodiment 2
[0071] Example 2. In Vitro Release of Local Anesthetics from Pharmaceutical Compositions
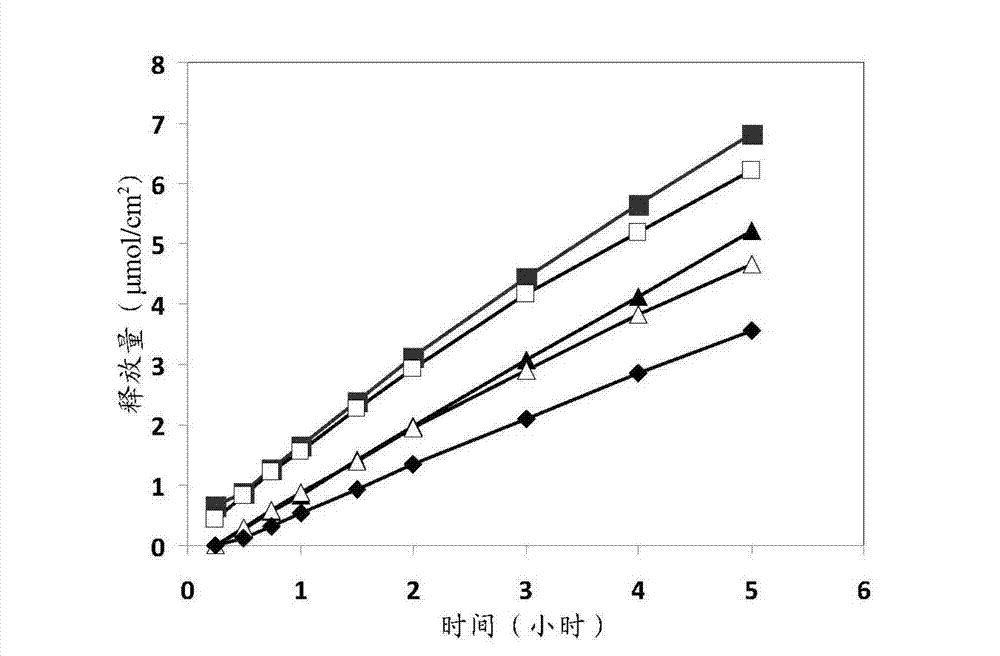
[0072] The release of lidocaine from the pharmaceutical composition containing 5% lidocaine prepared according to Example 1 was measured twice.
[0073] result in figure 1 shown in . A stable release of lidocaine from different drug formulations can be observed.
Embodiment 3
[0074] Example 3. Evaluation of Appearance at 37°C
[0075] A pharmaceutical composition containing 5% lidocaine or 5% ropivacaine was prepared according to Example 1. Examples of stabilizing compositions are shown in Table 4. After the preparation was completed, the formulation was equilibrated in a constant temperature water bath at 37°C.
[0076] Table 4. Appearance at 37°C and ejectability at room temperature (approximately 25°C) of stable lipid-based formulations containing lidocaine and ropivacaine
[0077]
[0078]
[0079]
[0080]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com