Silver-organic conductive ink for printed electronics
A conductive ink, organic technology, applied in the direction of ink, application, household appliances, etc., can solve the problems of clogging the nozzle, affecting the electrical performance of the wire, and difficulty in mass production of nano-silver ink, and achieves material saving, stable properties, and easy preparation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dissolve 0.01mol capric acid in a mixed solvent (45ml absolute ethanol, 5ml anhydrous ether), add 0.01mol triethylamine to form a salt. Then dissolve 0.01mol silver nitrate in 20ml mixed solvent (18ml absolute ethanol, 2ml anhydrous ether), and pour it into the previously configured solution. At this time, a large amount of decanoate silver-white precipitate will be produced, which is suction filtered, washed with ethanol and water, and dried in vacuum to obtain the final silver decanoate.
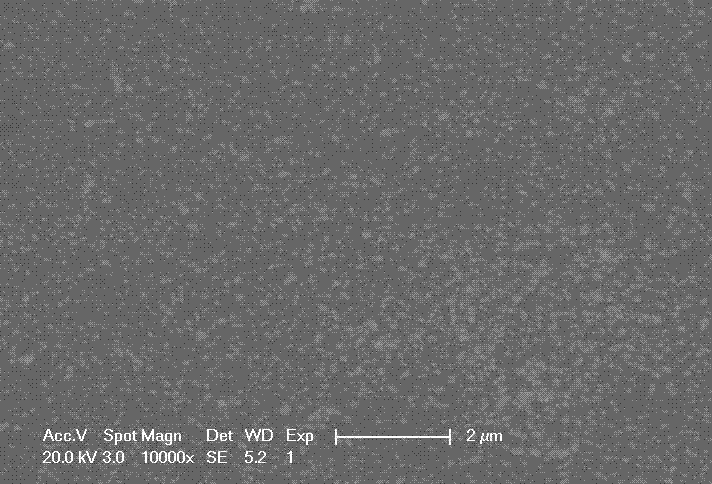
[0026] Add 0.005 mol of silver decanoate solid to 6.25 g of isopropanol, then add 0.01 mol of isopropylamine, and stir until all the solids are dissolved. A colorless and transparent solution is obtained.
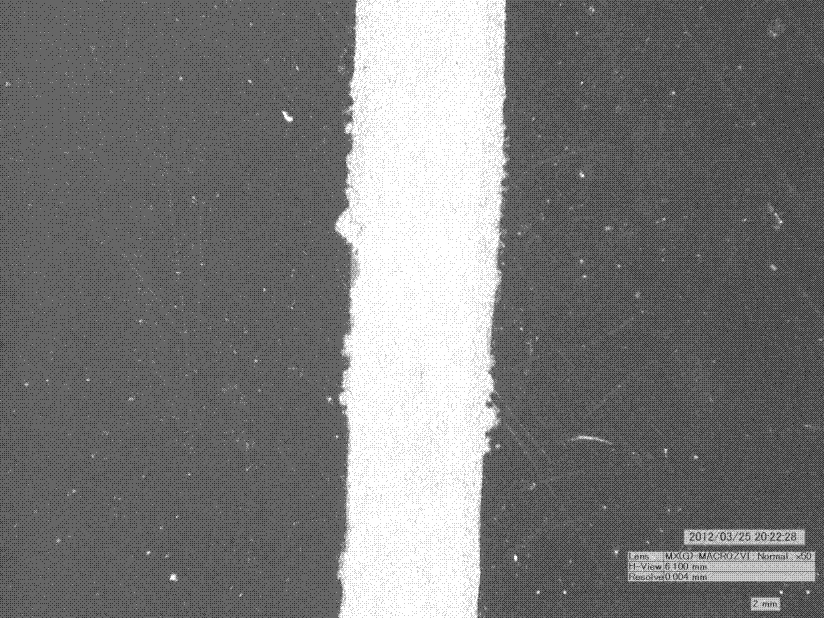
[0027] The prepared ink was spin-coated on glass, heat-treated in an oven at 200oC for 30 minutes, taken out to room temperature and placed in acetone for 10 minutes. After drying, the resistivity was measured to be 3.3E-6Ω·cm.
Embodiment 2
[0029] Dissolve 0.01mol formic acid in a mixed solvent (45ml absolute ethanol, 5ml anhydrous ether), add 0.01mol triethylamine to form a salt. Then dissolve 0.01mol silver nitrate in 20ml mixed solvent (18ml absolute ethanol, 2ml anhydrous ether), and pour it into the previously configured solution. At this time, a large amount of silver formate white precipitate will be produced, which is suction filtered, washed with ethanol and water, and dried in vacuum to obtain the final silver formate.
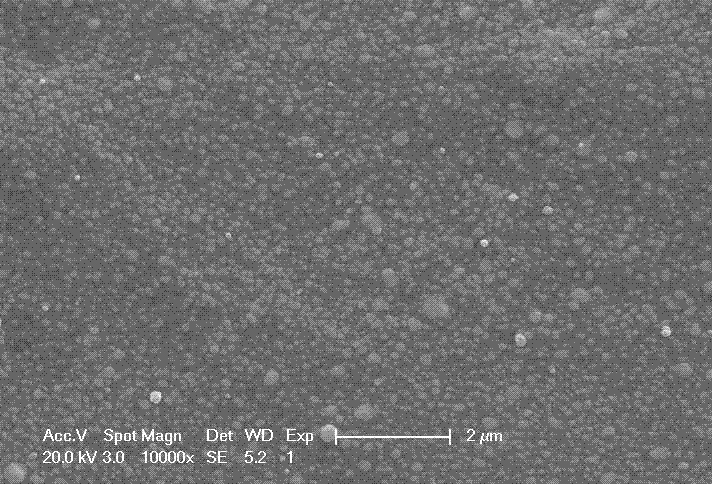
[0030] Add 0.005 mol of silver formate solids to 1.18 g of ethylene glycol, then add 0.01 mol of ethanolamine, and stir until all the solids are dissolved to obtain a colorless and transparent solution, which is configured as a 30% solid content ink.
[0031] The prepared ink was spin-coated on glass, heat-treated in an oven at 160°C for 30 minutes, and then placed in acetone for 10 minutes after taking it out to room temperature. After drying, the resistivity was measured at 8....
Embodiment 3
[0033] Dissolve 0.01mol of isooctanoic acid in a mixed solvent (45ml of absolute ethanol, 5ml of anhydrous ether), and add 0.01mol of triethylamine to form a salt. Then dissolve 0.01mol silver nitrate in 20ml mixed solvent (18ml absolute ethanol, 2ml anhydrous ether), and pour it into the previously configured solution. At this time, a large amount of silver isooctanoate will be produced, which will be filtered with suction, washed with ethanol and water, and dried in vacuum to obtain the final silver isooctanoate.
[0034] Add 0.005 mol of silver isooctanoate solid to 4.86 g of cyclohexanone, then add 0.01 mol of butylamine, and stir until all the solids are dissolved to obtain a yellow transparent solution, which is configured as a 20% solid content ink.
[0035] The prepared ink was spin-coated on glass, heat-treated in an oven at 200oC for 30 minutes, taken out to room temperature and placed in acetone for 10 minutes. After drying, the electrical resistivity was me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistivity | aaaaa | aaaaa |
| Resistivity | aaaaa | aaaaa |
| Resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com