Solid solution catalyst for hydrogen production by partial oxidation reforming of methane in coke oven gas
A technology for producing hydrogen from coke oven gas and reforming, which is applied in chemical instruments and methods, hydrogen, inorganic chemistry, etc., and can solve problems such as low vaporization heat and loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
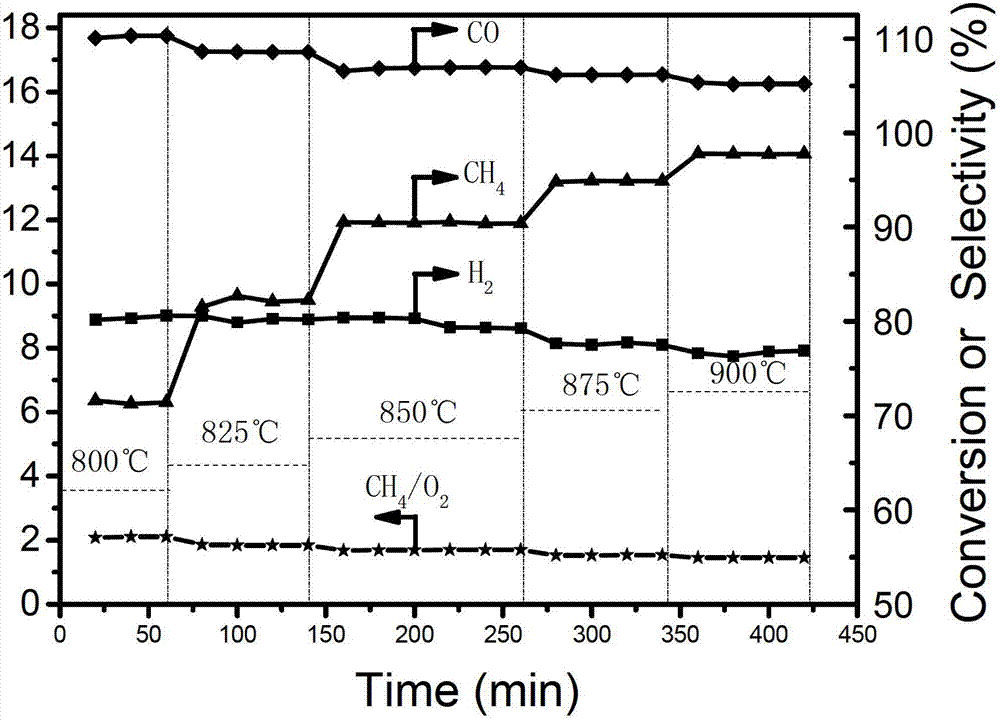
Image
Examples
Embodiment 1
[0031] Weigh 58.20596g of Co(NO 3 ) 2 ·6H 2 O, 58.158g of Mg (NO 3 ) 2 ·6H 2O was dissolved in 10ml of distilled water to form a mixed solution of cobalt nitrate and magnesium nitrate. Stir well and evenly, after mixing, stir and evaporate to dryness on a constant temperature magnetic stirrer at 90°C-100°C, and then put it in an oven and bake at 110°C for 3 hours. Then put it into a muffle furnace to program the temperature rise, and raise the temperature to 800° C. at a rate of 5° C. per minute and keep it warm for 5 hours. After the heat preservation, it is finally cooled to room temperature with the furnace. The calcined catalyst was ground into powder and pressed into tablets with a pressure of 30KN. After the tablet is pressed, it is crushed and sieved to make catalyst particles of 20-40 mesh.
Embodiment 2
[0033] Weigh 29.079g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 15ml of distilled water to form an aqueous solution of nickel nitrate. Then add 16.122g MgO to the nickel nitrate aqueous solution, stir well, mix well, stir fully at room temperature on a constant temperature magnetic stirrer for 12 hours, let it stand for 12 hours, filter, and then put the obtained solid in an oven at 110°C Bake for 3 hours. Then put it into a muffle furnace to program the temperature rise, and raise the temperature to 800° C. at a rate of 5° C. per minute for 5 hours, and then cool to room temperature with the furnace after the heat preservation. The calcined catalyst is then ground into powder and pressed into tablets with a pressure of 30KN. After the tablet is pressed, it is crushed and sieved to make catalyst particles of 20-40 mesh.
Embodiment 3
[0035] Weigh 46.5264g Ni(NO 3 ) 2 ·6H 2 O, 61.5384g Mg (NO 3 ) 2 ·6H 2 O was dissolved in 10ml of distilled water to form a mixed solution of nickel nitrate and magnesium nitrate. Stir well and evenly, after mixing, stir and evaporate to dryness on a constant temperature magnetic stirrer at 90°C-100°C, and then put it in an oven and bake at 110°C for 3 hours. Then put it into the muffle furnace to program the temperature rise, and raise the temperature to 800°C at a rate of 5°C per minute and keep it for 4 hours. room temperature. The calcined catalyst was ground into powder and pressed into tablets with a pressure of 30KN. After the tablet is pressed, it is put into the muffle furnace and the temperature is raised to 1200° C. at a rate of 5° C. per minute and kept for 10 hours. After cooling with the furnace, take out the diaphragm, crush and sieve to make catalyst particles of 20-40 mesh.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com