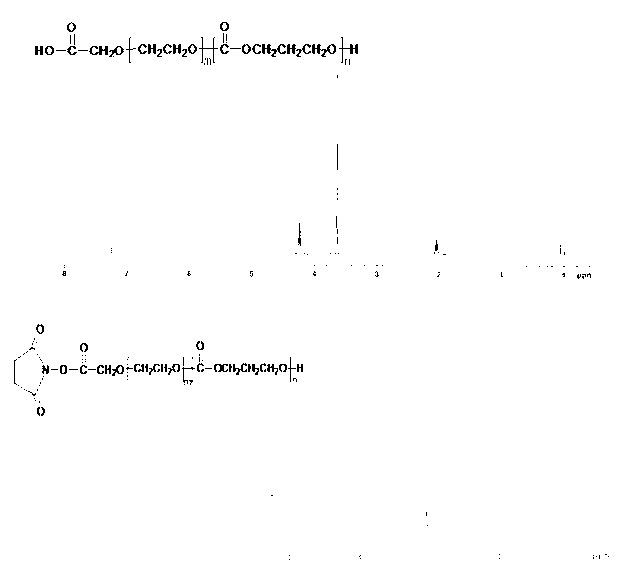Integrin receptor-targeted taxol nanocomposite and preparation method thereof
A technology of integrin receptors and nanocomposites, applied in the field of biomedicine, can solve problems such as organ toxicity and side effects, reduce drug therapeutic index, etc., and achieve the effect of improving therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Amphiphilic block copolymer HOOC-PEG-PTMC with an active group carboxyl group and a preparation method thereof, comprising the steps of:
[0035] 0.95g of HOOC-PEG (molecular weight is 3500) and 2.00g of dimethylene carbonate (TMC) were placed in a dry flask, and the toluene solution of stannous octoate was added as a catalyst. Under nitrogen protection, 140± React at 1° C. for 48 hours; the final product was dissolved in 5 ml of chloroform, and purified by precipitation with 3 times the amount of hexane. The purified product was vacuum-dried at 40°C for 24 hours to obtain the amphiphilic block copolymer HOOC-PEG-PTMC with an active group carboxyl group (stored at -20°C sealed); gel permeation chromatography (GPC) Analyze the purity of the product to 1 The molecular weight of the product was calculated from the peak area ratio of the proton in the methylene group in the H-NMR spectrum.
Embodiment 2
[0037] Amphiphilic copolymer modified with RGD peptide and preparation method thereof, comprising steps:
[0038] (1) Preparation of NHS-PEG-PTMC by activating the carboxyl group with NHS
[0039] Weigh HOOC-PEG-PTMC (0.5g, 0.053mmol), DCC (0.022g, 0.106mmol, 2×excess) and NHS (0.0122g, 0.106mmol, 2×excess) described in Example 1, dissolve In 5ml of chloroform, react at room temperature under nitrogen protection for 24 hours; after the reaction is completed, remove the solvent under reduced pressure, and precipitate with cold ether; dry the precipitate in vacuum to constant weight to obtain NHS-PEG-PTMC; 1 H-NMR spectrum is verified to product;
[0040] (2) Preparation of c(RGDyK)-PEG-PTMC with cyclic RGD peptide (c(RGDyK)) modified amphiphilic copolymer
[0041] Take the NHS-PEG-PTMC (50mg, 0.0053mmol) in the above step (1) and dissolve it in 1ml of dimethylformamide to obtain solution A; take 6.3mg (0.01mmol) of c(RGDyK) peptide and dissolve it in 0.1 M HEPES to obtain so...
Embodiment 3
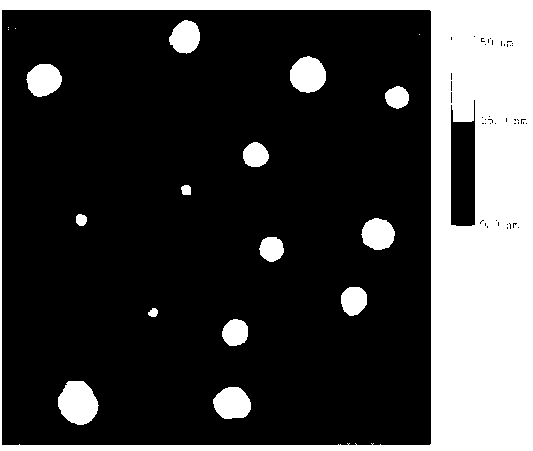
[0043] Weigh 35 mg of MePEG-PTMC, 5 mg of c(RGDyK)-PEG-PTMC and 2 mg of paclitaxel, add 1 ml of dichloromethane, and ultrasonically dissolve the carrier material and drug fully. The solution was added to 5ml of 0.6% sodium cholate solution, and the ice-bath probe was sonicated with the ultrasonic power of 200 W, the ultrasonic time of 5 s, the ultrasonic interval of 5 s, and the number of ultrasonic waves 15 times. Obtain colostrum after ultrasonication, add the colostrum dropwise to 25ml of 0.3% sodium cholate solution, stir magnetically at 100r / min, after stirring for 1min, remove dichloromethane by rotary evaporation at 37°C, and obtain the entrapped Paclitaxel targeting integrin receptor nanocomplexes.
[0044] The particle size measurement results show that: the average particle size of the nanocomposite is 50.3±4.7nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com