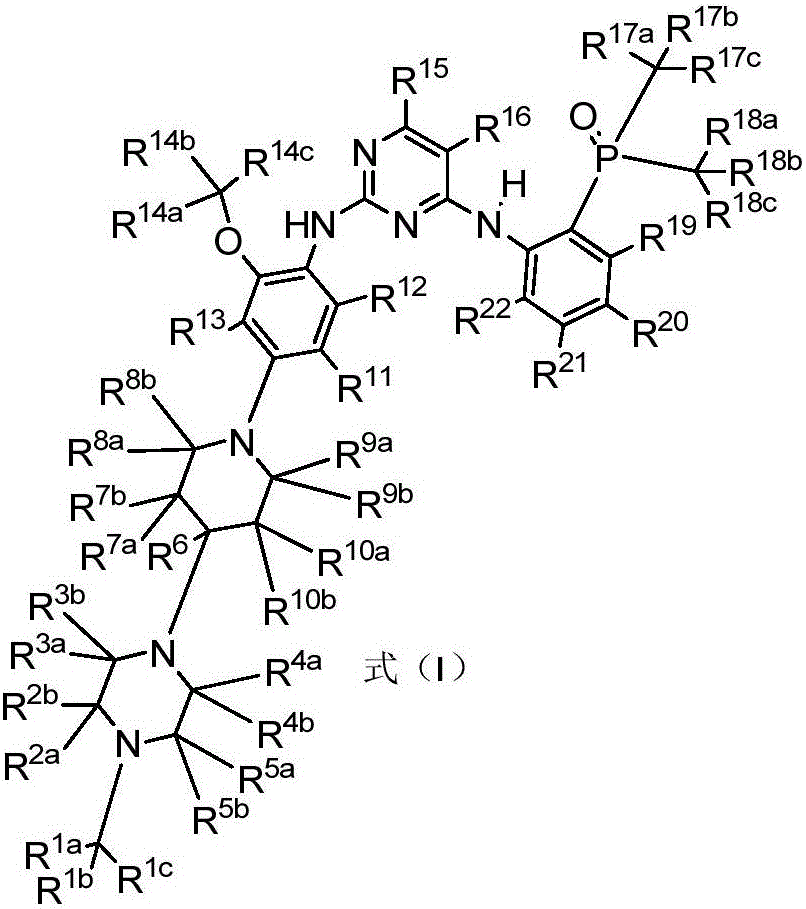
Diaminopyrimidine compound and composition containing same
A technology of diaminopyrimidine and compounds, applied in the field of medicine, can solve problems such as visual impairment, elevated liver transaminase levels, and gastrointestinal side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
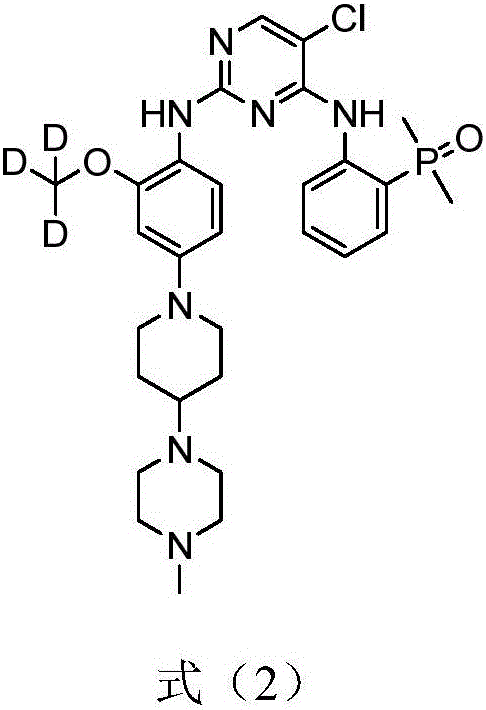
[0095] Prepare 5-chloro-N according to the following synthetic route 4 -[2-(Dimethylphosphoryl)phenyl]-N 2 -{2-d3-methoxy-4-[4-(4-methylpiperazin-1-yl)-piperidin-1-yl]phenyl}pyrimidine-2,4-diamine (synthesized below Compound 9 in the scheme):
[0096]
[0097] Prepared using the following steps:
[0098] (1) Preparation of compound 2:
[0099] Add 30mL of acetone to a 100mL single-necked bottle, and add 5-fluoro-2-nitrophenol (2.0g, 12.7mmol), anhydrous potassium carbonate (3.5g, 25.4mmol), deuteroiodomethane (2.4g, 16.5mmol), heated to 60°C and kept stirring for 2h. Cool to room temperature, spin evaporate the acetone, add 20 mL of water to the residue, extract with ethyl acetate (30 mL x 3), combine the organic layers, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to obtain 2.0 g of white solid, with a yield of 90%.
[0100] 1 H NMR (300MHz, CDCl 3 )(δ / ppm)8.00-7.95(m,1H),6.83-6.71(m,2H), LC-MS(APCI):m / z=175.2(M+1) + ,95%.
[0101] (2) P...
Embodiment 2
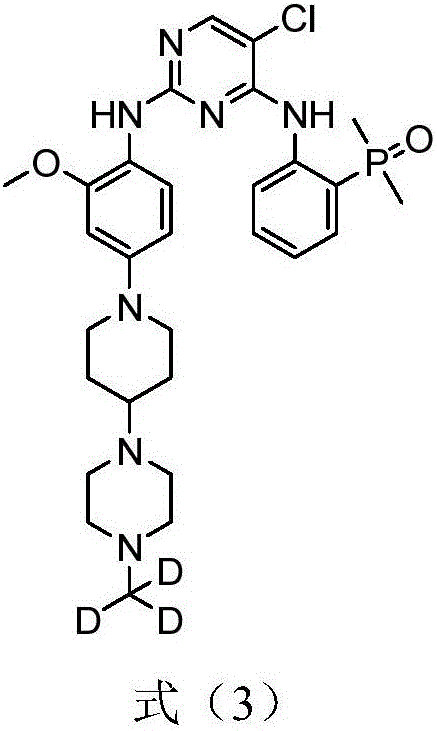
[0113] Prepare 5-chloro-N according to the following synthetic route 4 -[2-(Dimethylphosphoryl)phenyl]-N 2 -{2-methoxy-4-[4-(4-d3-methylpiperazin-1-yl)-piperidin-1-yl]phenyl}pyrimidine-2,4-diamine (synthesized below Compound 15 in the scheme):
[0114]
[0115] Include the following steps:
[0116] (1) Preparation of Compound 10:
[0117] Add N,N-dimethylformamide (10mL) into a 100mL single-necked flask, and add 4-fluoro-2-methoxynitrobenzene (2g, 11.8mmol) and piperidin-4-one hydrochloride in sequence under stirring (2.23g, 16.5mmol), anhydrous potassium carbonate (4.88g, 35.4mmol), the reaction mixture was warming up to 80 ° C, N 2 react overnight under atmosphere. Cooled to room temperature, poured into ice water (80mL), precipitated a large amount of yellow solid, filtered, dissolved in DCM (100mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to give 2.2g of yellow solid, yield 74.6%.
[0118] LC-MS(APCI):m / z=251.2(M+1) + ; 1...
Embodiment 3
[0131] Prepare 5-chloro-N according to the following synthetic route 4 -[2-(Dimethylphosphoryl)phenyl]-N 2 -{2-methoxy-4-[4-(4-methylpiperazin-1-yl)-4-d-piperidin-1-yl]phenyl}pyrimidine-2,4-diamine (under Compound 20 in the above synthetic route):
[0132]
[0133]
[0134] Include the following steps:
[0135] (1) Preparation of compound 16:
[0136] Add 10 mL of deuterated methanol into a 50 mL single-necked flask, add 1-(3-methoxy-4-nitrophenyl)piperidin-4-one (0.25 g, 1 mmol) under stirring in an ice-water bath, and slowly Add deuterated sodium borohydride (42mg, 1mmol), ice water bath N 2 Stir the reaction under atmosphere for 5 min, add heavy water (2 mL) to quench the reaction, and stir at room temperature for 30 min, add water (30 mL) and ethyl acetate (30 mL) successively, separate the organic layer, extract the aqueous layer with ethyl acetate (30 mL x 2), concentrate , the residue was redissolved in ethyl acetate (50 mL), washed with saturated brine (20 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com