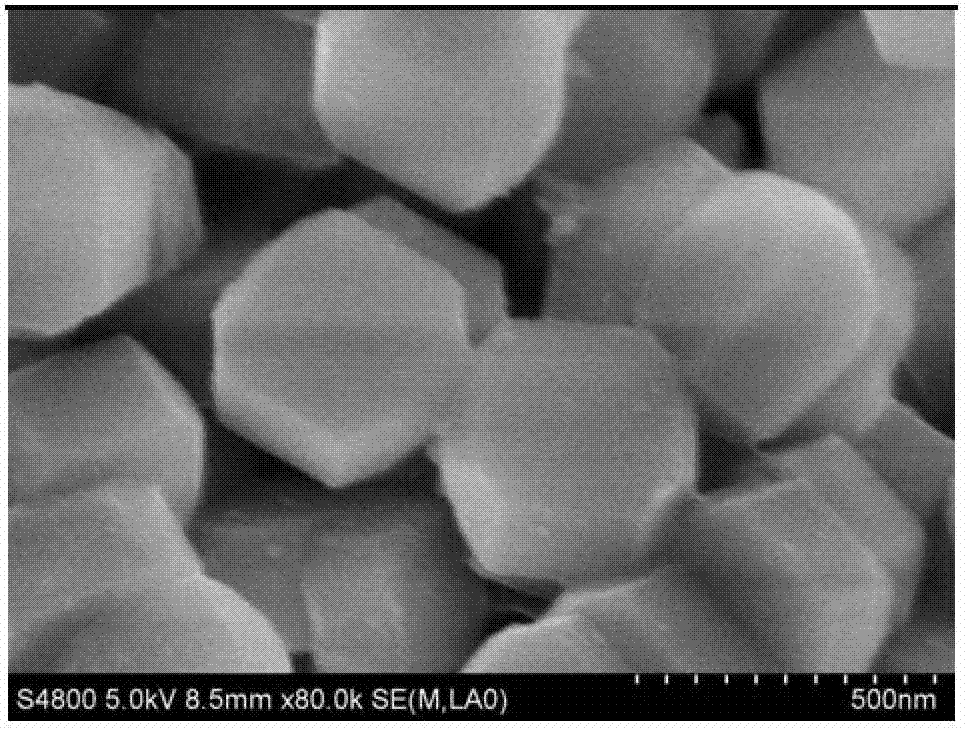
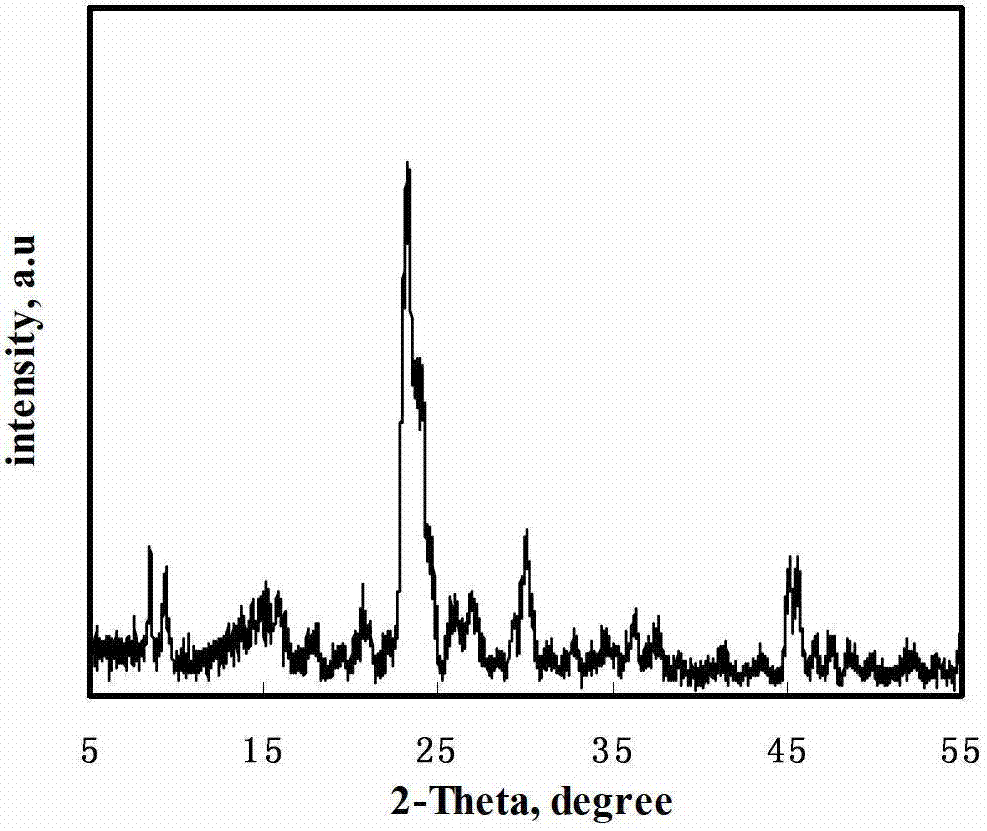
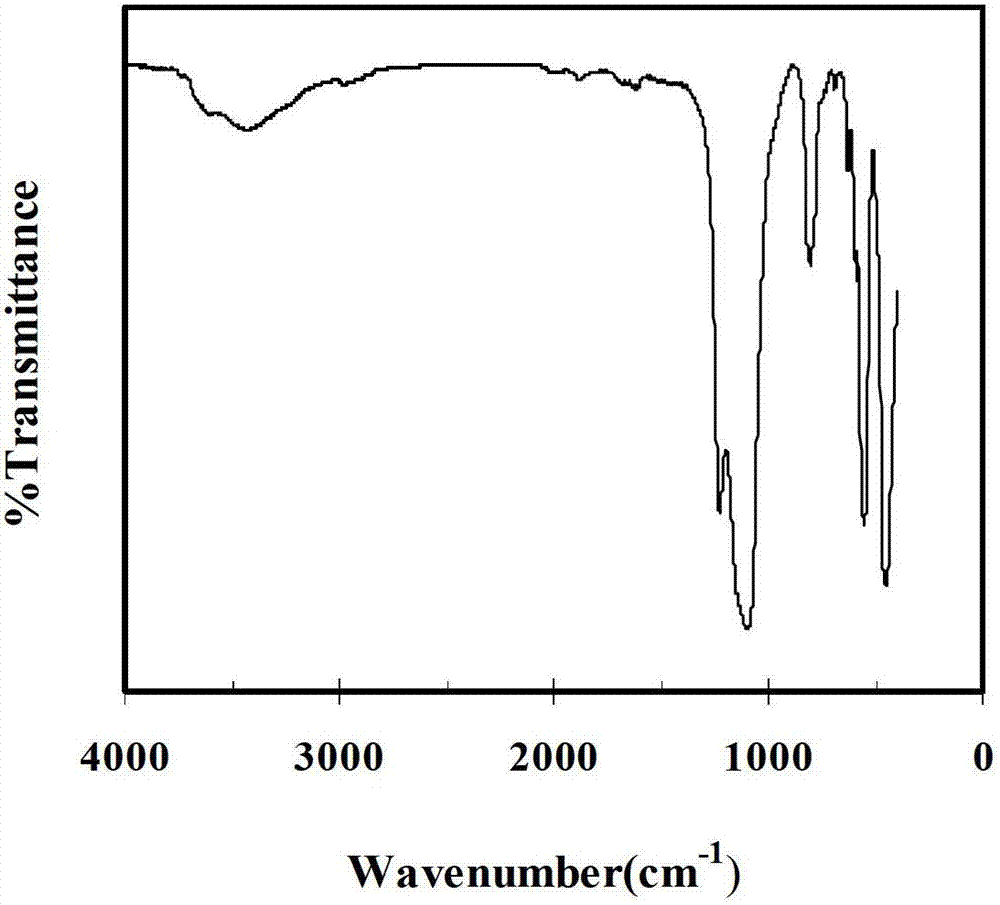
Method for preparing organic lactone
A technology of organic and lactones, which is applied in the field of catalysis for the preparation of organic lactones by oxidation of organic ketones, can solve the problems of low efficiency, difficult catalyst recycling, low catalyst activity and selectivity, etc., and achieves simple preparation, good industrial application prospects, Catalyst cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 0.5 g of the above-mentioned synthesized hexagonal prism-shaped Co-MFI catalyst, 10 g of cyclopentanone, 10 g of benzaldehyde, and 200 ml of carbon tetrachloride in sequence into a three-necked glass bottle, and feed oxygen at a flow rate of 100 ml / min, after reacting at 40 DEG C under stirring for 4 hours, the gas chromatography-mass spectrometry analysis result shows that the product is the target product valerolactone, and the gas chromatography analysis result shows that the selectivity and yield of valerolactone are respectively 100 % and 99%. After the catalyst was recycled 10 times, the selectivity and yield of valerolactone were still 100% and 99.2%, respectively.
Embodiment 2
[0038] Add 0.2 g of the Co-MFI catalyst with hexagonal prism shape synthesized above, 10 g of cyclohexanone, 20 g of butyraldehyde, and 200 ml of 1,2-dichloroethane into a three-necked glass bottle in sequence, and then inject oxygen, oxygen Flow velocity is 200 milliliters / minute, after reacting at 80 ℃ under stirring for 1 hour, gas chromatography-mass spectrometry analysis result shows that product is target product caprolactone, and gas chromatography analysis result shows the selectivity and recovery of caprolactone. The rates are 100% and 99.5%, respectively.
Embodiment 3
[0040] Add 0.8 g of the above-mentioned hexagonal prism-shaped Co-MFI catalyst, 10 g of 2-methylcyclohexanone, 40 g of p-chlorobenzaldehyde, and 200 ml of acetonitrile into a three-necked glass bottle in sequence, and then introduce oxygen at a flow rate of Be 400 milliliters / minute, after reacting at 30 ℃ under stirring for 9 hours, gas chromatography-mass spectrometry analysis result shows that product is target product 2-methyl caprolactone, and gas chromatography analysis result shows that 2-methyl caprolactone The selectivity and yield of lactone are both 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com