Method for preparing self-assembly ketoprofen liposome by electrostatic spinning technology
An electrospinning technology and ketoprofen lipid technology are applied in the field of preparation of pharmaceutical liposomes, which can solve the problems of limited application, poor stability of liposomes, etc., and achieve simple operation, less time-consuming, good degradability and The effect of biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Put the mixture of chloroform and N,N-dimethylacetamide (DMAc) at a volume ratio of 4:1 into the reaction vessel;
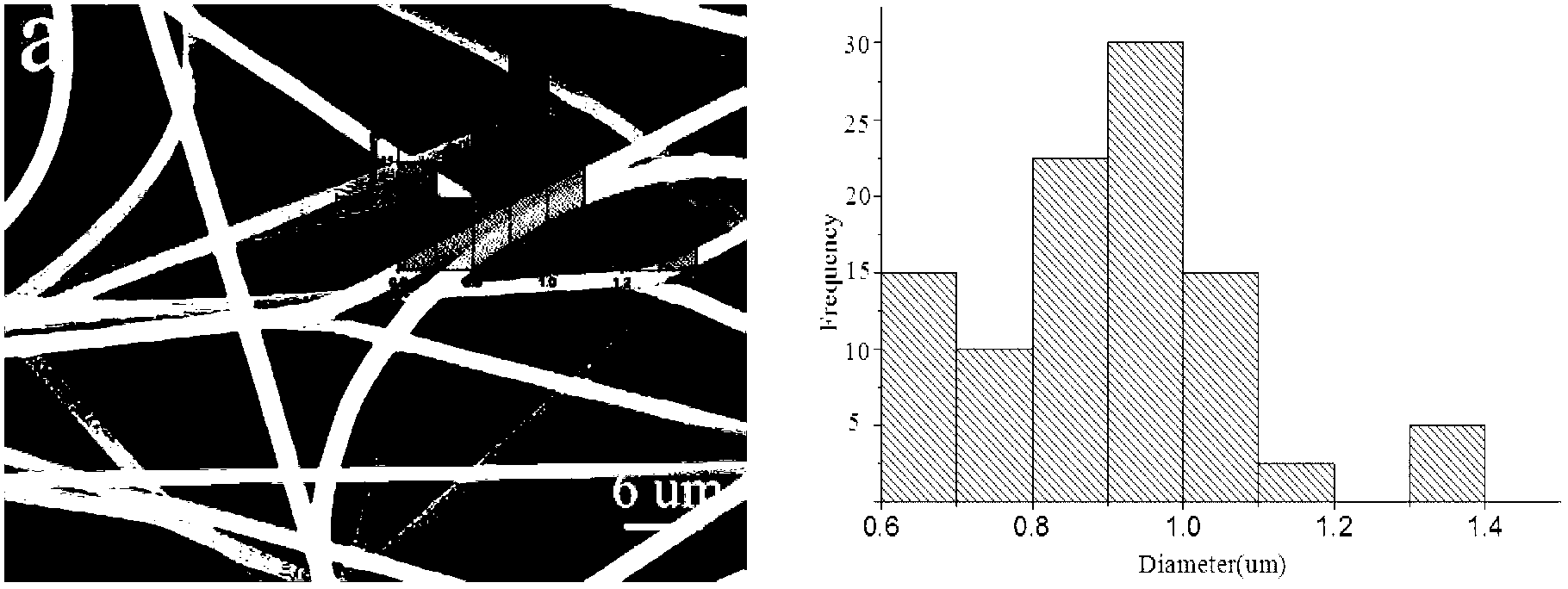
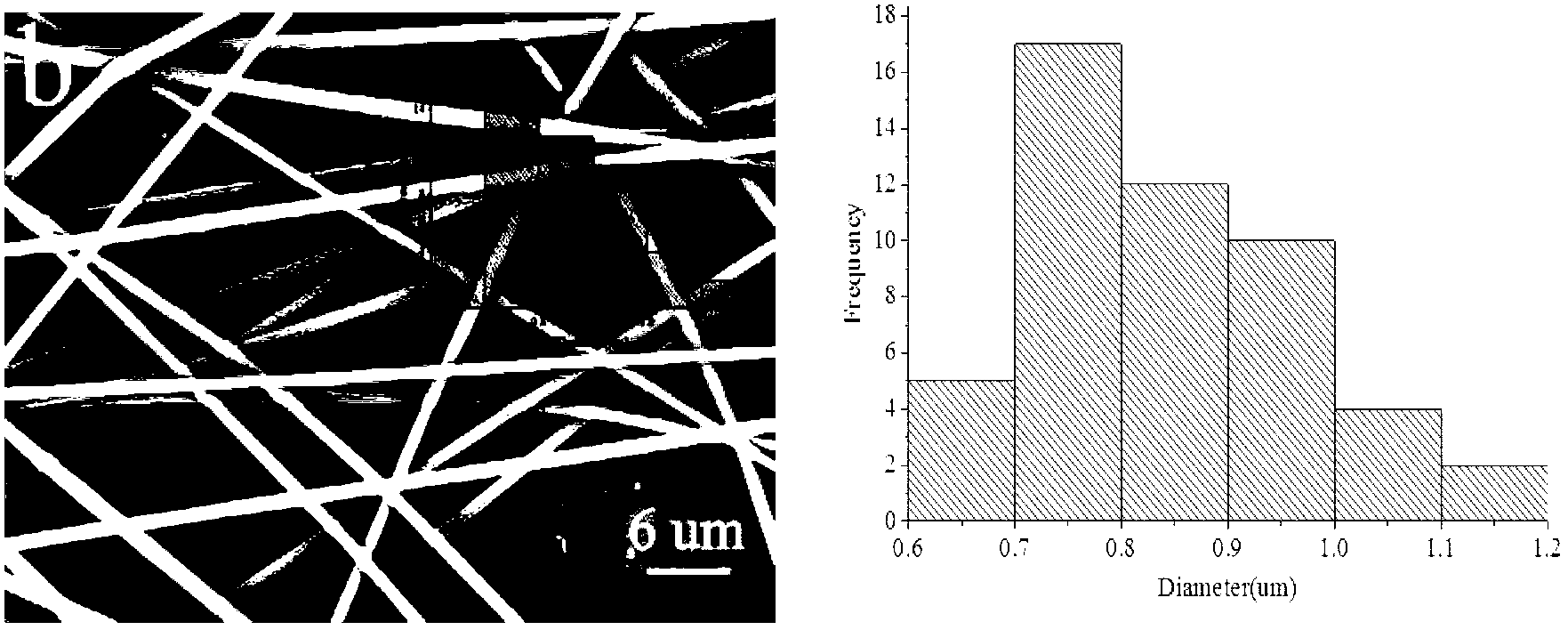
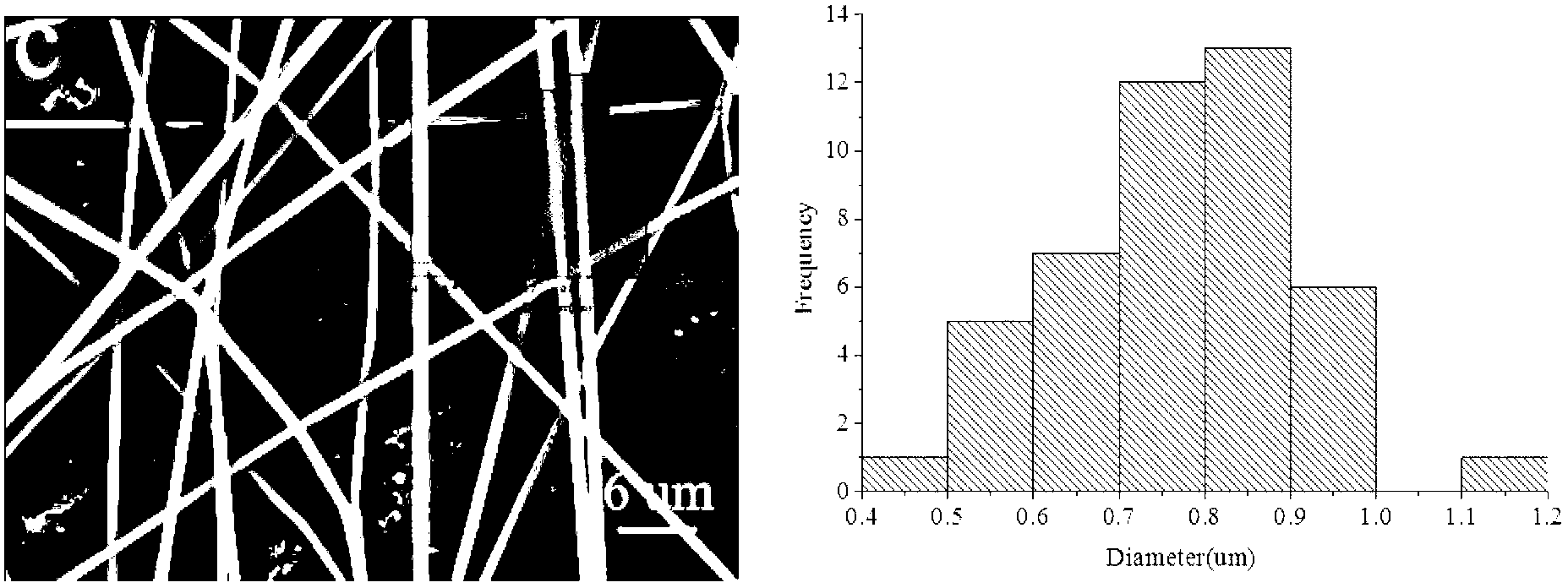
[0031] (2) Slowly add the weighed PVP, PC and ketoprofen powder into the above-mentioned reaction vessel under stirring conditions, and continue to stir for 1.5h to completely swell; wherein, the content of PVP in the mixture of chloroform and DMAc is fixed at 10 % (w / w), the content of PC in the mixture of chloroform and DMAc was fixed at 5% (w / w), and the mass ratio of ketoprofen to PC was 0:100, 3:25, 5:25, 10 :25, 15:25;
[0032] (3) Place the Erlenmeyer flask in a shaker and shake it at 25°C for 24 hours until it is completely dissolved. The polymer solution is transparent, and it is degassed by ultrasonic treatment for 15 minutes to obtain a spinning solution;
[0033] (4) Use a 5ml syringe (the inner diameter of the needle is 0.5mm) to extract the spinning solution, fix it on the electrospinning device, set the static voltage to 14kv, the recei...
Embodiment 2
[0037] (1) Take 10ml of liposome suspensions with different drug-to-lipid ratios, dilute to 25ml with methanol, and ultrasonically treat for 30 minutes to break the emulsion. Take 0.5ml from them and dilute to 100ml with double distilled water. Under the condition of a wavelength of 260nm, Measure its absorbance to calculate the total content of ketoprofen;
[0038] (2) Take 10ml of liposome suspensions with different drug-to-lipid ratios, put them in dialysis bags (MWCO: 3500), and seal them;
[0039] (3) At the same time, take 200ml of double-distilled water as the external dialysis fluid, stir at room temperature with a stirring speed of 150rpm for 24 hours, and measure the absorbance of the external dialysis fluid at a wavelength of 260nm to calculate the content of free ketoprofen;
[0040] (4) Calculate its encapsulation rate according to the following formula:
[0041] E.E.(%)=(1-free ketoprofen amount / total ketoprofen amount)×100, the ketoprofen encapsulation efficiency...
Embodiment 3
[0043] (1) Take 10ml of liposome suspension with a drug-to-lipid ratio of 5:25, put it in a dialysis bag (MWCO:3500), and seal it;
[0044] (2) At the same time, take 200ml of double-distilled water as the external fluid for dialysis, and shake at 37°C with a stirring speed of 150rpm for 24h to remove free ketoprofen;
[0045] (3) Replace the external dialysis fluid with buffer solutions of different pH values, keep the temperature at 37°C, shake at 150rpm, and take samples from it with a sampler at 0h, 0.5h, 1h, 2h, 4h, 6h, 12h, 24h, 48h and 72h 3ml (2 parallel operations), measure the absorbance at 260nm at each time point, and calculate the release percentage with the drug content in the 0h liposome as 100%. The release curves of ketoprofen from liposomes under different buffers are as follows: Figure 7 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com