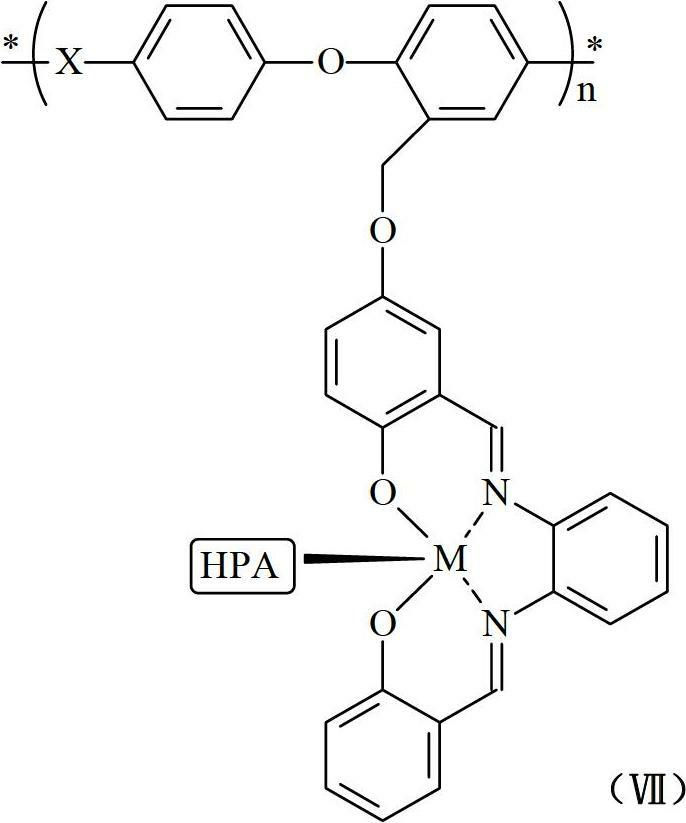
Proton exchange membrane made from high polymer-metal complex-heteropoly acid material and preparation method thereof
A metal complex, proton exchange membrane technology, applied in the field of fuel cells, can solve the problems of no proton conductivity, inability to meet battery working conditions, and imperfect composite membrane performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
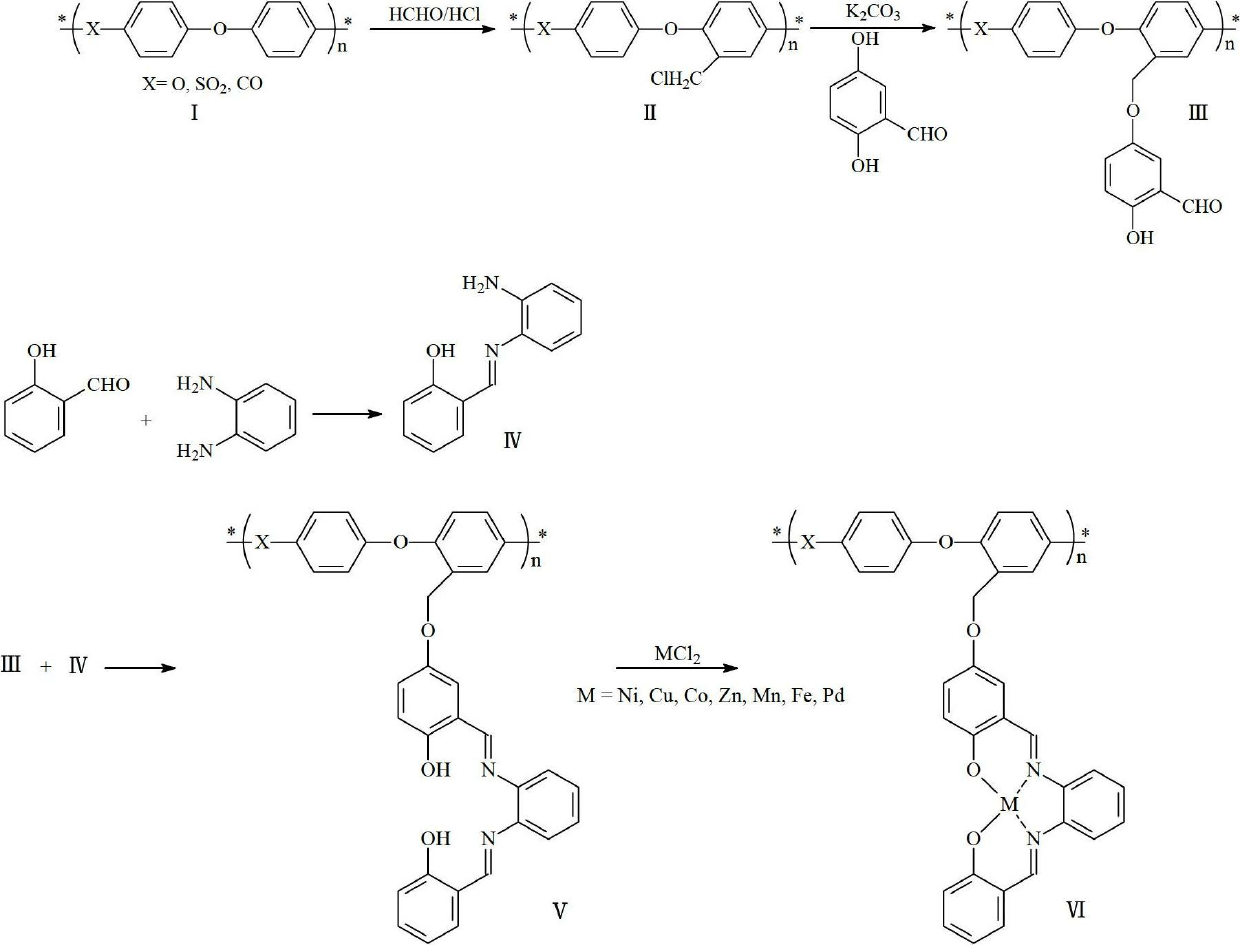
[0036] (1) Dissolve 5g of polyphenylene ether in 100ml of DMF, add 1g of polyoxymethylene and 5mL of concentrated hydrochloric acid, react at 30°C for 5h, pour into ice water, filter, wash with water, and vacuum dry at 60°C to obtain product A (chlorine methylated polyphenylene ether).
[0037] (2) Dissolve the above-prepared product A and 2g of 2,5-dihydroxybenzaldehyde in DMF, add 2g of anhydrous potassium carbonate, stir and react at 100°C for 4h, concentrate under reduced pressure and drain the solvent, add water and stir, wash to remove inorganic salt, dried in vacuo to obtain product B (polyphenylene ether compound with phenolic ether attached to the side chain).
[0038] (3) Dissolve 1.22g salicylaldehyde and 1.08g o-phenylenediamine in absolute ethanol, stir for 4 hours, and filter to obtain product C (salicylaldehyde mono-o-phenylenediamine solid).
[0039] (4) Dissolve product B and product C in toluene, reflux to remove water, concentrate, and filter with suction t...
Embodiment 2
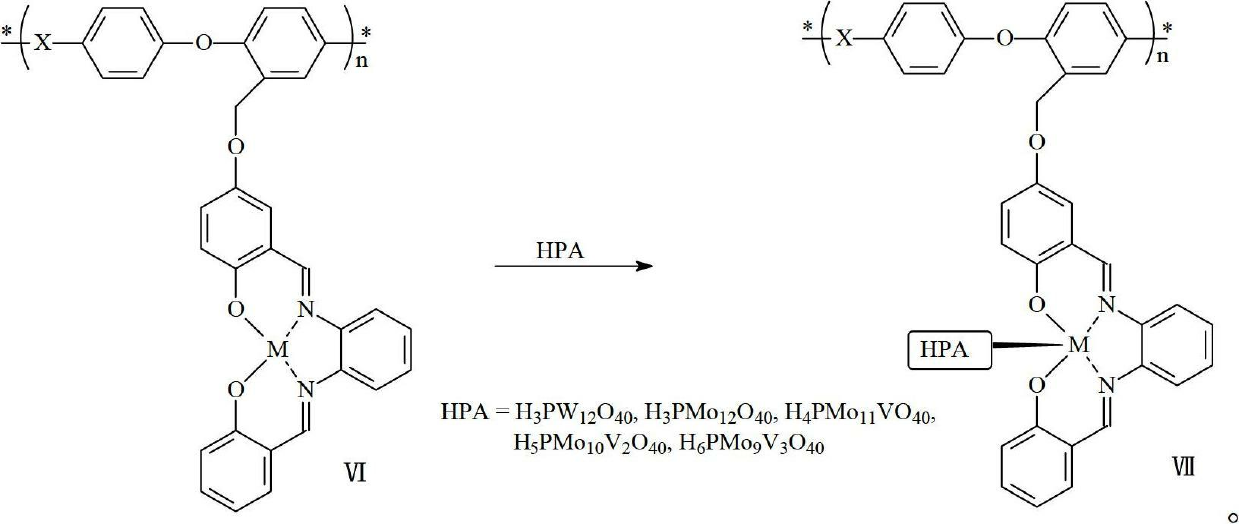
[0044] The operation steps are similar to Example 1, except that in step (6), phosphotungstic acid is replaced by phosphomolybdic acid to obtain a proton exchange membrane containing polyphenylene ether-manganese complex-phosphomolybdic acid polymer compound.
Embodiment 3
[0046] The operation steps are similar to Example 1, except that in step (5), zinc chloride is used to replace manganese chloride to obtain a proton exchange membrane containing polyphenylene ether-zinc complex-phosphotungstic acid polymer compound.
[0047] The physical and chemical properties of the polymer-metal complex-heteropolyacid ternary coupling proton exchange membrane material or membrane obtained in the above 3 examples are listed in the following table:
[0048]
[0049]
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| osmotic coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com