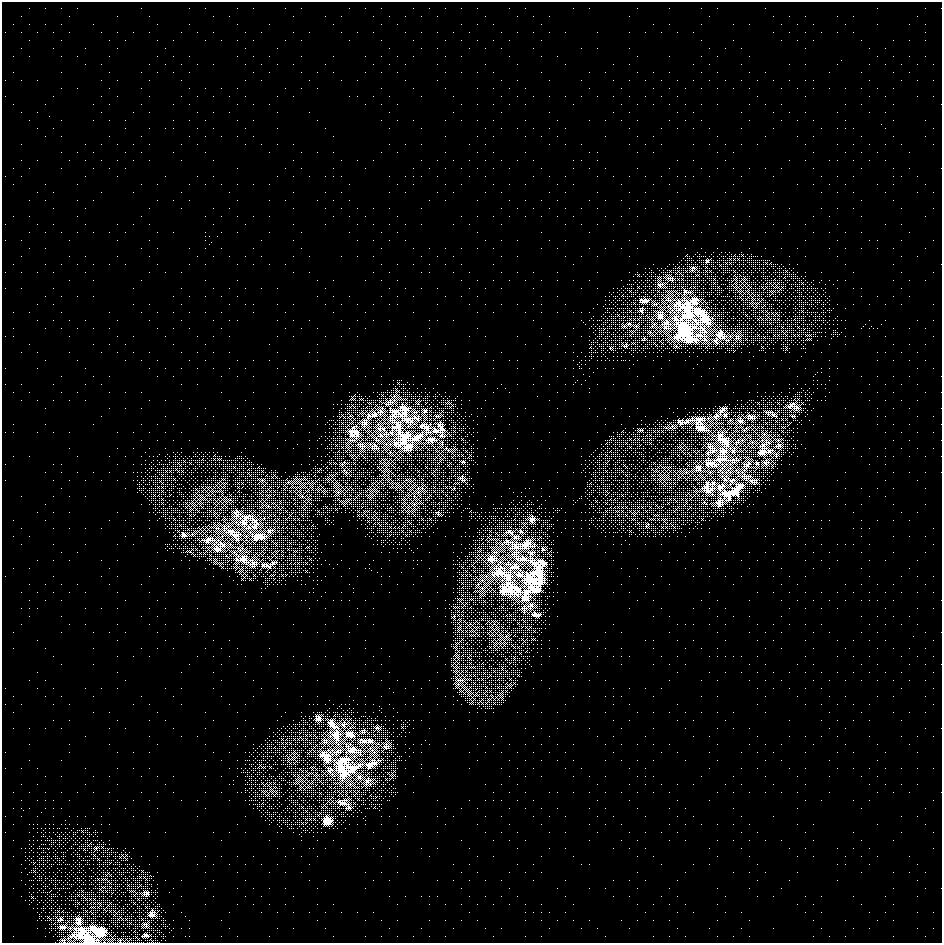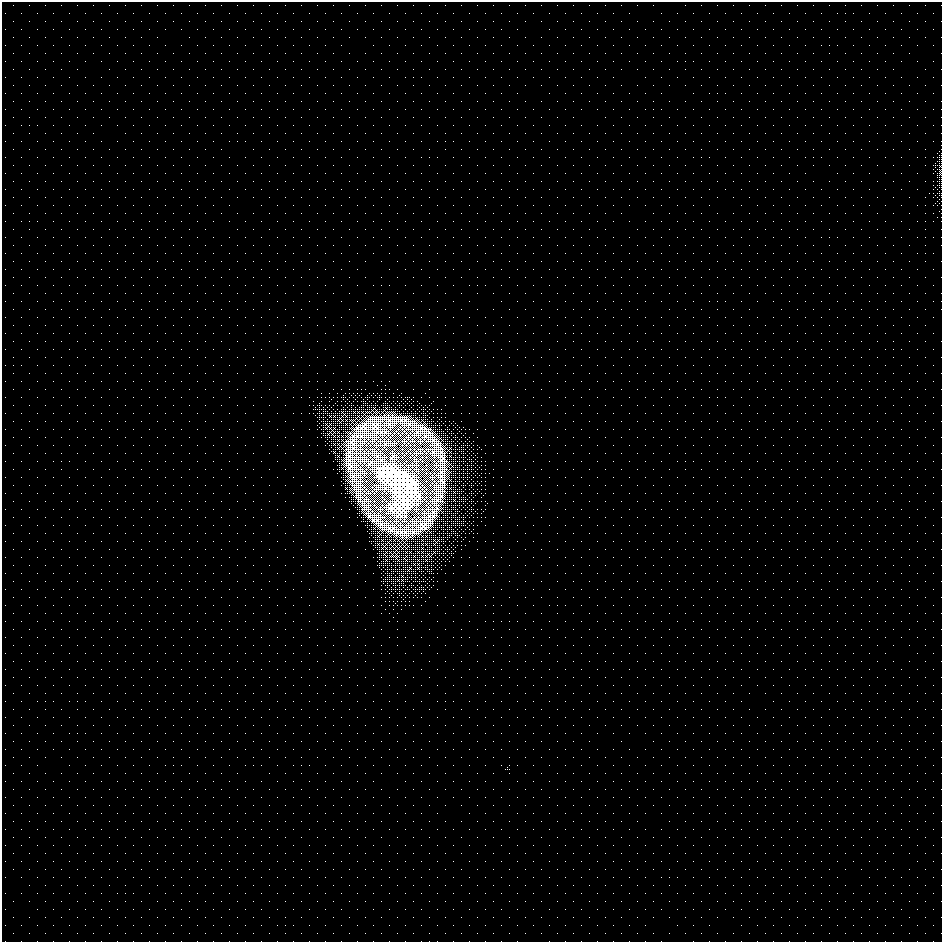4-N substituted anthracene pyridone fluorescent dye and preparation method and application thereof
A fluorescent dye and anthrapyridone technology, applied in the field of fluorescent dyes, can solve problems such as difficulty in penetrating ultraviolet light, difficulty in fluorescence detection, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. Synthesis of Dye A and Live Cell Fluorescence Imaging
[0038] (1) Synthesis of Dye A
[0039]
[0040] 4-Bromo-N-methylanthrapyridone (13 g, 38.2 mmol), ethylenediamine (5 mL, 83.3 mmol), potassium carbonate (5.3 g, 38.2 mmol) and anhydrous copper sulfate (1.0 g, 6.3 mmol) were dissolved in 150mL of ethylene glycol methyl ether, refluxed for 20h, TLC detected the raw material 4-bromo-N-methylanthrapyridone, filtered to remove insolubles, and the filtrate was evaporated under reduced pressure to obtain a dark red solid, and the crude product was washed with ethyl acetate Washed (3×50 mL) and dried to obtain 11.0 g of the target product with a yield of 89%.
[0041] 1 H NMR (400MHz, DMSO) δ 10.47 (t, J=9.6Hz, 1H, NH), 8.55 (d, J=7.9Hz, 1H, ArH), 8.36 (d, J=7.7Hz, 1H, ArH) , 8.23 (t, J=6.0Hz, 1H, NH), 7.94 (d, J=9.7Hz, 1H, ArH), 7.87-7.70 (m, 3H, ArH, CH), 7.55 (d, J=9.7Hz) , 1H, ArH), 5.69 (s, 1H, CH), 5.35 (s, 1H, CH), 3.77 (s, 3H, CH) 3 ), 3.66-3.5...
Embodiment 2
[0044] Example 2. Synthesis of Dye B and Live Cell Fluorescence Imaging
[0045] (1) Synthesis of Dye B
[0046]
[0047] The synthetic method of dye B is similar to that of dye A, and the main raw materials used are 4-bromo-N-methylanthrapyridone and ethanolamine. The crude product was separated by silica gel column, yield: 83%
[0048] 1 H NMR (400MHz, DMSO) δ 10.53 (s, 1H, NH), 8.53 (d, J=3.2 Hz, 1H, ), 8.37 (d, J=5.6 Hz, 1H, ArH), 7.76-7.94 (m , 4H, ArH, CH), 7.41(d, J=3.2Hz, 1H), 5.02(s, 1H), 3.74(m, 5H), 3.51(s, 2H). MS(TOF MS ES+) calculated for[ C 19 H 17 N 2 O 3 ] + : 321.1234, measured: 321.1238.
[0049] (2) Observe the staining of HeLa in living cells by compound B under a confocal laser scanning microscope:
[0050] Compound B was added to the cultured HeLa cells to a final concentration of 5 μM in 2 mL of cell culture medium. Incubate in a cell incubator at 37°C, 5% CO2 for 30 minutes. Then, 0.01M PBS was shaken and rinsed for 5 minutes × 3, and then...
Embodiment 3
[0051] Example 3. Synthesis of Dye C and Live Cell Fluorescence Imaging
[0052] (1) Synthesis of Dye C
[0053]
[0054] The synthetic method of dye C is similar to that of dye A, and the main raw materials used are 4-bromo-N-methylanthrapyridone and N,N-dimethylpropanediamine. The crude product was separated on a silica gel column, yield: 60%.
[0055] 1 H NMR (400MHz, CDCl3) δ 10.53 (s, 1H, NH), 8.52 (d, J=6.8Hz, 1H, ArH), 8.24 (d, J=7.8Hz, 1H, ArH), 7.82-7.62 ( m, 4H, ArH, CH), 7.30 (d, 1H, ArH), 3.88 (s, 3H, CH3), 3.52 (dd, J=12.5, 6.8Hz, 2H, CH2), 2.53 (t, J=7.0 Hz, 2H, CH2), 2.33(s, 6H, CH3), 2.01(dd, J=14.1, 7.0Hz, 2H, CH2). MS(TOF MS ES+) calculated for [C22H24N3O2] + : 362.1863, measured: 362.1869.
[0056] (2) Observe the staining of HeLa in living cells by compound C under a confocal laser scanning microscope:
[0057] Compound C was added to the cultured HeLa cells to a final concentration of 5 μM in 2 mL of cell culture medium. Incubate in a cell incuba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com