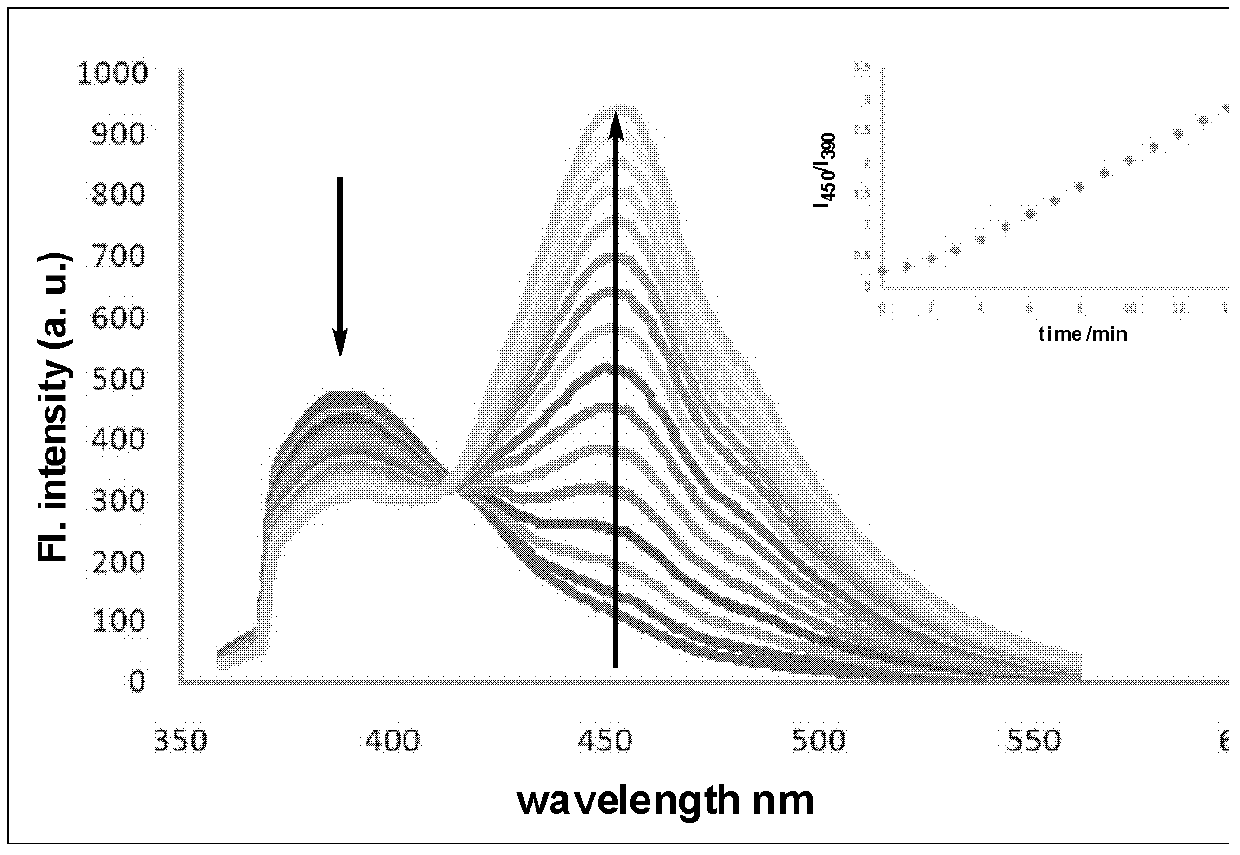
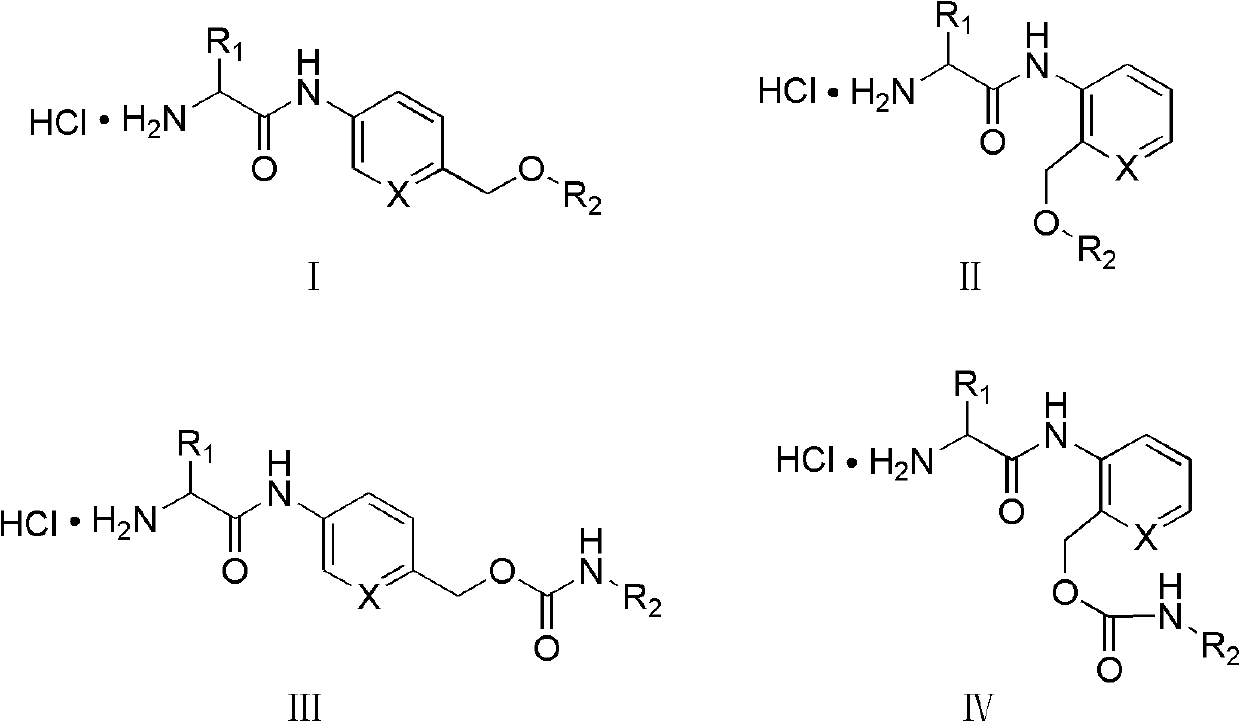
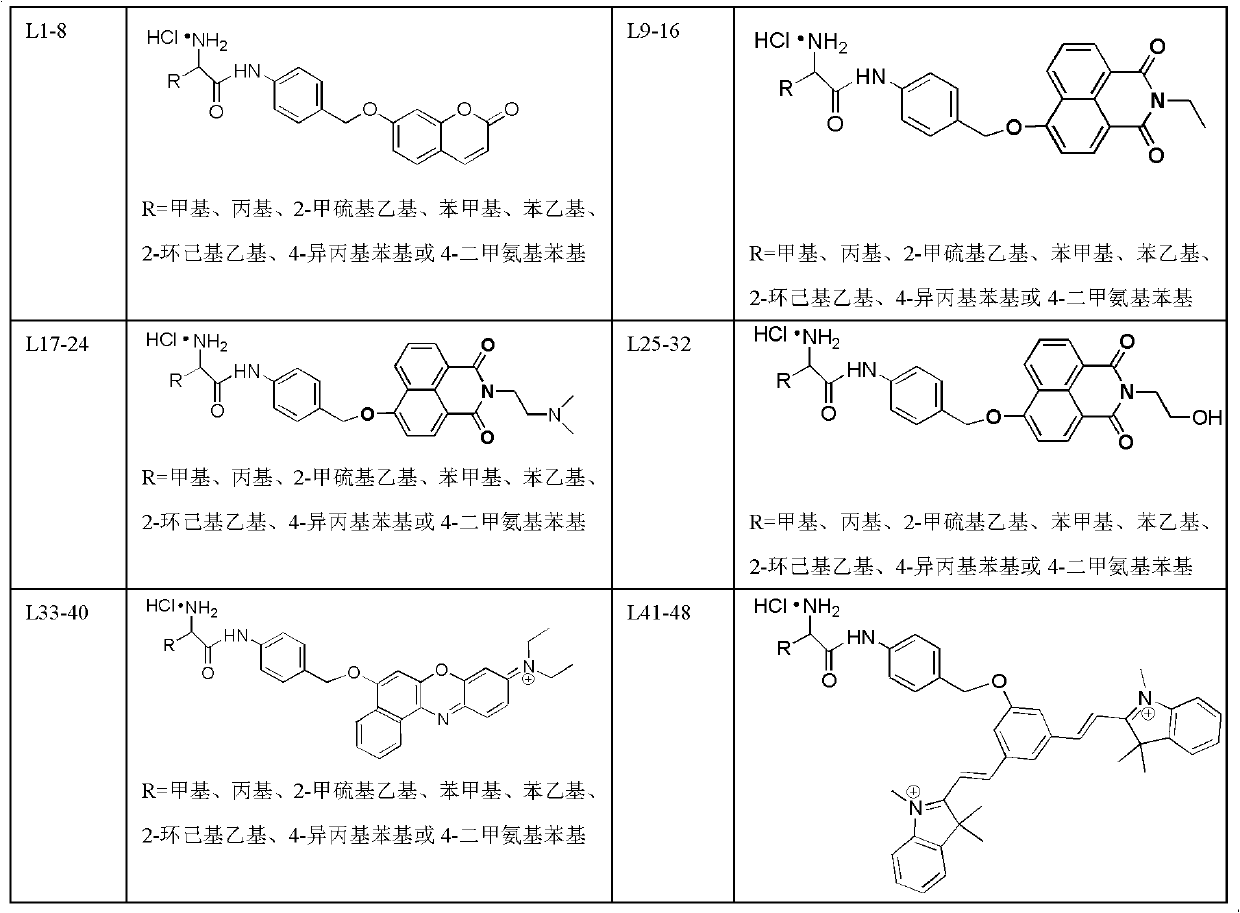
Amino acid-fluorophore compound and application thereof
A compound, fluorophore technology, applied in the field of medicine, can solve the problems of low sensitivity, high cost, long test time and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Example 1: Preparation of 2-amino-4methylthio-N-(4-(2-oxo-2hydro-chromen-7-yloxy)phenyl)butyramide hydrochloride【L3】
[0023] Synthetic route 1
[0024]
[0025] Preparation of Intermediate 1:
[0026] Dissolve 14.9g of methionine in 110mL of 1M sodium hydroxide solution, place in an ice bath, and slowly add 50mL of anhydrous THF solution containing 2118g of di-tert-butyl carbonate dropwise, while using 1M sodium hydroxide solution to control the pH of the reaction solution at about 9, about After 1 hour of dropwise addition, stir for 1 hour in an ice bath, remove the ice bath, and stir at room temperature for 15 hours. During the whole process, the pH of the system was kept at around 9. After the reaction was completed, THF was distilled off, washed with petroleum ether (100mL×3), the aqueous phase was adjusted to pH 2-3 with saturated citric acid solution, and extracted with ethyl acetate (100mL×3). Dry over magnesium sulfate with water and concentrate to obtain...
example 2
[0040] Example 2: Preparation of 2-amino-N-(2-((2-oxo-2hydro-chromen-7-yloxy)-methyl)phenyl)pentanamide hydrochloride【L98】
[0041] Synthetic route 2
[0042]
[0043] Preparation of Intermediate 7:
[0044] Dissolve 1.2g Boc-L-norvaline in 50mL dichloromethane, add 0.75g HOBT, 1.05g EDCI, stir at room temperature for 15min, add 0.62g o-aminobenzyl alcohol, and stir at room temperature for 10 hours. The reaction solution was washed with saturated citric acid (50 mL×3), saturated sodium bicarbonate (50 mL×3), and saturated brine (50 mL×1). Dry over anhydrous magnesium sulfate overnight. Concentrate and recrystallize from ethyl acetate-n-hexane to obtain 0.97 g of a white solid with a yield of 60% and a melting point of 110.0-122.3°C.
[0045] Preparation of Intermediate 8:
[0046] Using intermediate 7 as raw material, according to the preparation method of intermediate 5, intermediate 8 was obtained as a brown oil with a yield of 80%. Preparation of Intermediate 9:
...
example 3
[0051] Example 3: 2-amino-N-(2-((2-oxo-2hydro-chromen-7-yloxy)-methyl)pyridin-3-yl)propionamide hydrochloride [L337 】Preparation
[0052] Synthetic route 3
[0053]
[0054] References for the synthesis of intermediates 10 and 11 [(a) Beyermann, M.; Bienert, M.; Niedrich, H; Carpino, L.A.; Sadat-Aalaee, D. Rapid continuous peptide synthesis via FMOC amino acid chloride coupling and 4-( aminomethyl)piperidine deblocking.J Org Chem, 1990, 55, 721-728. (b) Carpino, L A.; Xia, J.S.; El-Faham, A. 3-Hydroxy-4-oxo-3,4-dihydro- 5-azabenzo-1, 2, 3-triazene. J Org Chem, 2004, 69, 54-61.].
[0055] Preparation of intermediate 12:
[0056] Dissolve 2.67g of intermediate 11 and 3mL of pyridine in 50mL of dioxane, add 0.63g of intermediate 10, reflux for 1 hour and cool to room temperature. Concentrate, dissolve with 200 mL ethyl acetate, wash with saturated sodium bicarbonate (50 mL×3), and saturated brine (50 mL×1). Dry over anhydrous magnesium sulfate overnight. Concentrate and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com