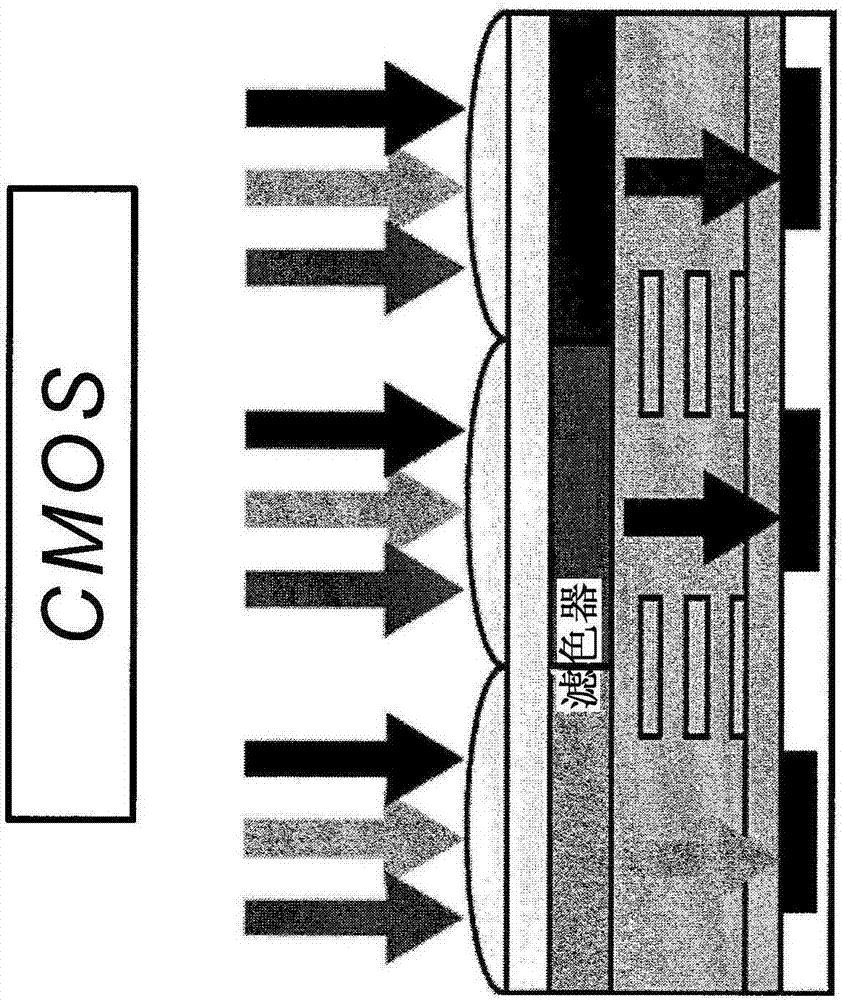
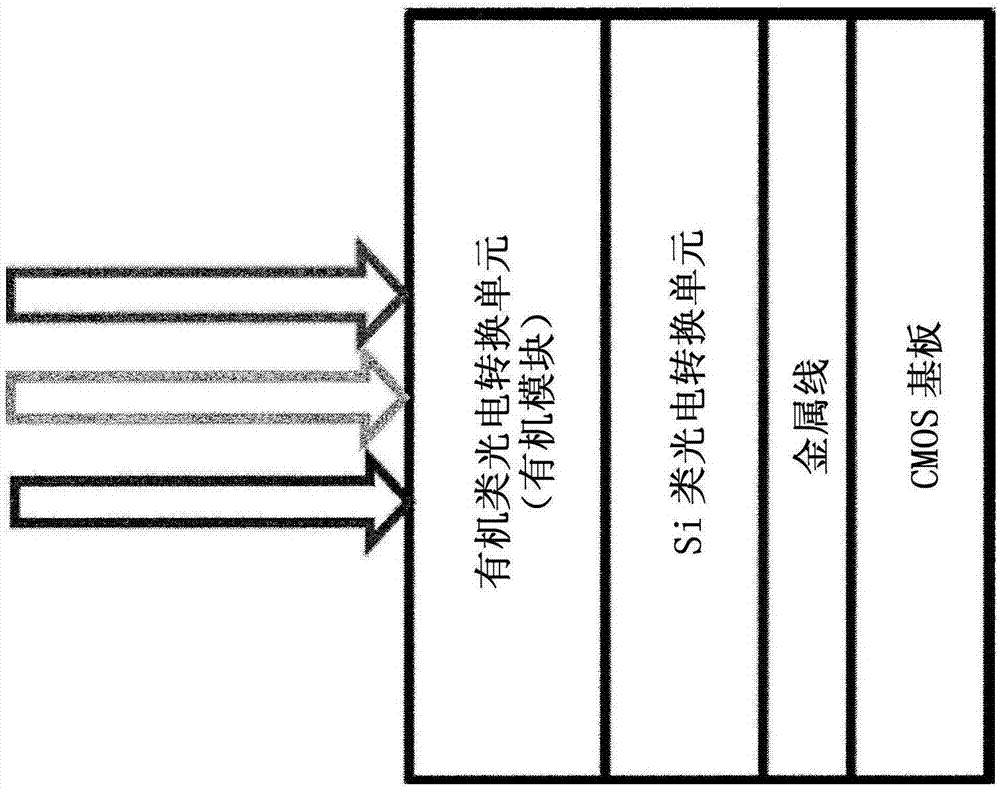
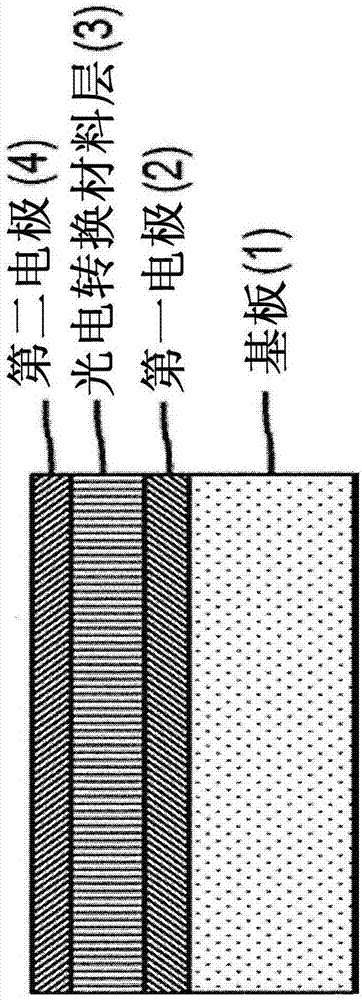
Specific n and p active materials for organic photoelectric conversion layers in organic photodiodes
A technology of N-R2 and naphthalene diimide, applied in photovoltaic power generation, organic chemistry, circuits, etc., can solve the problems of spatial resolution, light collection and utilization efficiency reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0414] Example 1: Naphthalene diimide (NDI) based materials
[0415] Naphthalene diimide (NDI) NDI1 has the following chemical structure:
[0416]
[0417] exist Figure 5 In the schemes shown, general synthetic routes for the synthesis of the materials are reported.
[0418] via HPLC, 1 Material NDI1 was characterized by H NMR, mass spectrometry, TG and DSC. Figure 6 to Figure 9 The data are shown in .
[0419] The absorption spectrum shows a very low optical density in the visible range ( Figure 10 ). Electron mobility up to 10 -7 up to 10 -5 cm 2 / Vs( Figure 11 ).
[0420]NDI1 was used as acceptor material in combination with quinacridone (QD) and tributylquinacridone (BQD) as donors for the following constructs:
[0421] ITO / 8nm HG01 / 120nm QD&NDI1(1:1) / 3,5nmNBPhen / 100nm AlSiCu / 100nm LiF
[0422] ITO / 5nm MoO3 / 8nm HG01 / 120nm QD&NDI1(1:1) / 3,5nmNBPhen / 100nm AlSiCu / 100nm LiF
[0423] ITO / 8nm HG01 / 120nm BQD&NDI1(7:3) / 3,5nmNBPhen / 100nm AlSiCu / 100nm LiF
[0424...
Embodiment 2
[0430] Example 2: Further naphthalene diimide (NDI) based materials
[0431] Naphthalimides (NDI) NDI20-26, NDI 28-29 and NDI35-38 have the following chemical structures:
[0432]
[0433]
[0434] exist Figure 15 In the schemes shown, general synthetic routes for the synthesis of the materials are reported.
[0435] NDI materials show absorption maxima in the range of 379 to 385 nm ( Figure 16 ).
[0436] Figure 17 to Figure 1 The energy levels are shown in 9.
[0437] NDI35 was used as n-buffer material in devices of DTT2, DTT9, DTT10 or DTT11 respectively with F6SubPcOC6F5 in the following configuration:
[0438] LiF 150nm / AlSiCu 100nm / NDI35 10nm / DTT9:F6SubPcOC6F5(1:1) 200nm / ST116310nm / ITO / Glass.
[0439] The device is characterized, for example, by measuring the action spectrum at 0V and -1V. The results are shown in FIGS. 20 and 21 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com