Novel n-type quinoid-structure small molecule and application thereof in organic photoelectric devices
A small molecule, n-type technology, applied to a new n-type quinoid structure small molecule and its application in organic optoelectronic devices, can solve the problems of insufficient absorption spectrum and low absorption coefficient, etc., to improve the absorption coefficient and electrons. Mobility, photocurrent enhancement, and effects of cell device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A representative synthetic route is as follows:
[0037]
[0038]
[0039] (1) Dibromonaphthalimide was synthesized according to the method disclosed in the literature [Journal of Materials Chemistry C, 2015, 3(34):8904-8915.].
[0040] Put 1.8g of dibromonaphthalimide, 210mg of pyromellitidine hydrochloride, and 200mg of potassium acetate in a 100ml two-necked bottle, react at 85°C for 36 hours under nitrogen protection, and add deionized water to quench the reaction. The product was extracted with dichloromethane and purified by column chromatography to obtain 1.2 g of product A with a yield of 75%.
[0041] Take 313mg of the product A obtained in the previous step, add 50mg of orthodiphenol, 33mg of potassium carbonate and react at 85°C for 12 hours under the protection of nitrogen, cool to room temperature, add 1g of lead dioxide to the reaction mixture, and overnight at 85°C. After cooling and filtering, the product was purified by column chromatography to o...
Embodiment 2
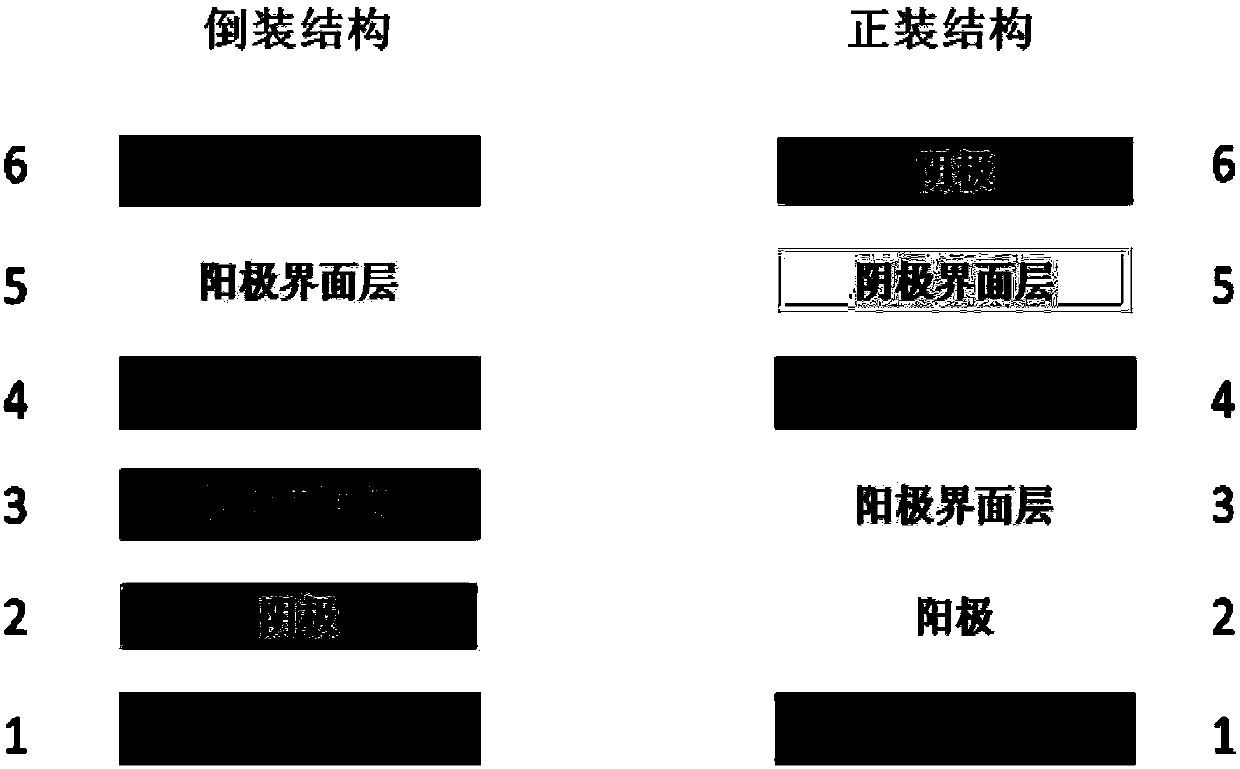
[0046] The quinone structure small molecules S1, S2, and S3 synthesized in Example 1 are used as electron acceptors in organic photovoltaic devices (ITO cathode / cathode interface layer / active layer / anode-machine interface layer / anode).
[0047] Pre-cut the ITO conductive glass with a square resistance of 20 ohms / cm2 into 15mm×15mm square pieces. Use acetone, special detergent for micron-sized semiconductors, deionized water, and isopropanol to clean ultrasonically in sequence, blow nitrogen whistle, and place in a constant temperature oven for later use. Spin-coat a layer of PFN-Br with a thickness of 5nm on ITO, and then spin-coat the active layer materials PTB7-Th / S1, PTB7-Th / S2,
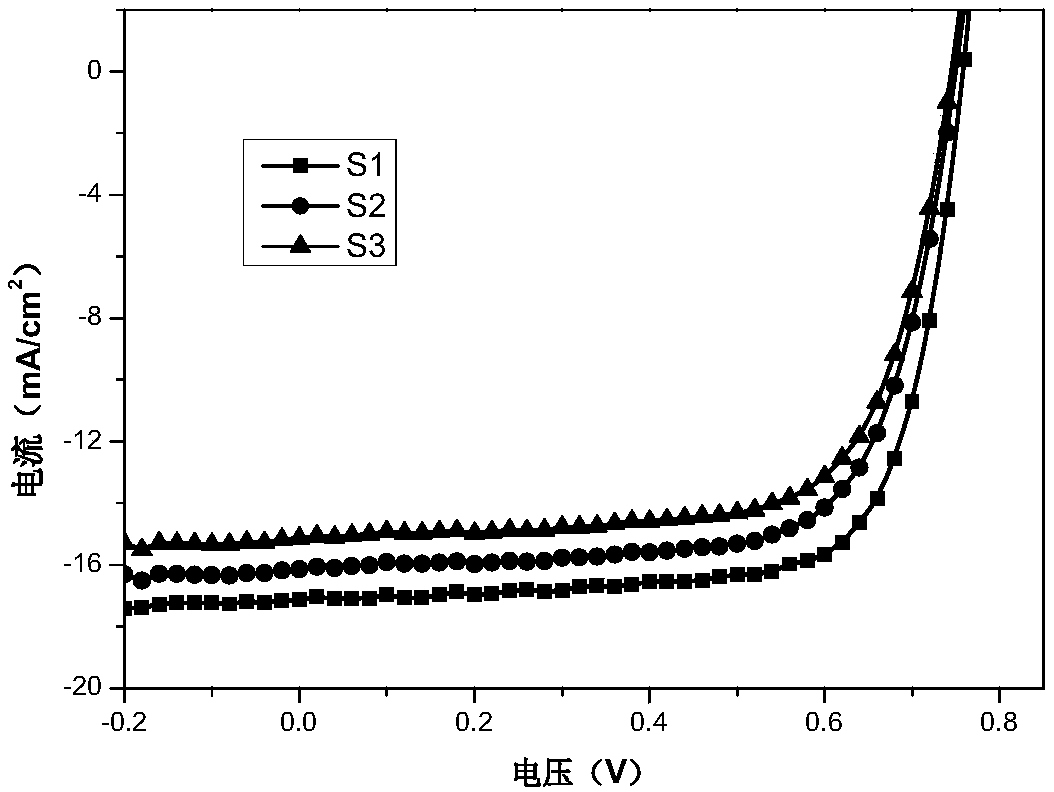
[0048] PTB7-Th / S3 with a thickness of 110nm and finally evaporated MoO 3 and Al electrodes. All preparations were carried out in a glove box under a nitrogen atmosphere. The current-voltage curves of the fabricated flip-chip devices are as follows: image 3 The relevant data are listed in Tabl...
Embodiment 3
[0050] The quinone structure small molecules S1, S2, and S3 synthesized in Example 1 are used as electron acceptors in organic photovoltaic devices (ITO anode / anode interface layer / active layer / cathode interface layer / cathode).
[0051] Pre-cut the ITO conductive glass with a square resistance of 20 ohms / cm2 into 15mm×15mm square pieces. Use acetone, special detergent for micron-sized semiconductors, deionized water, and isopropanol to clean ultrasonically in sequence, blow nitrogen whistle, and place in a constant temperature oven for later use. Spin-coat a layer of PEDOT:PSS with a thickness of 20 nm on the ITO, and then spin-coat the active layer materials PTB7-Th / S1, PTB7-Th / S2, and PTB7-Th / S3 with a thickness of 100 nm. Then spin-coat a layer of PFN-Br with a thickness of 5nm, and finally evaporate Al electrodes. All preparations were carried out in a glove box under a nitrogen atmosphere. The current-voltage curves of the prepared positive battery devices are as follow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com