Novel crystal form D of Retigabine and preparation method thereof
A retigabine and crystal form technology, applied in the field of medicinal chemistry, can solve problems such as uncertain bioavailability, difficult quality control, non-specific crystal form, etc., to avoid pollution and changes in drug properties, reduce production links, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
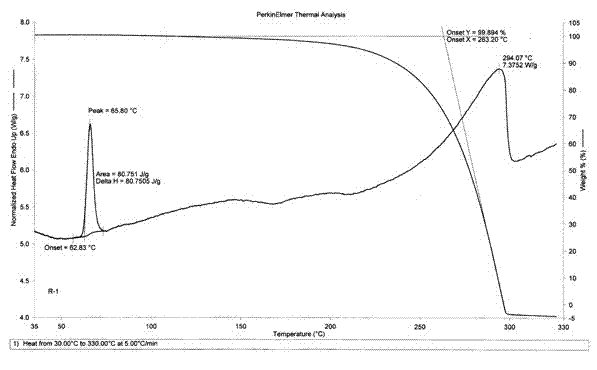
Image
Examples
Embodiment 1
[0028] Add 2kg of retigabine and 8L of diethyl ether into the dissolution tank, stir at room temperature until the solids are completely dissolved, filter, pump the filtrate into the crystallization tank, cool to -10°C to 0°C, stir and crystallize for 4 hours, and filter with a centrifuge. Washed with ether for 3 times, spin-dried, and dried under reduced pressure to obtain 1.83 kg of new crystal form D of retigabine.
Embodiment 2
[0030] Add 2kg retigabine and 8L diethyl ether into the dissolving tank, stir at room temperature until the solids are completely dissolved, filter, pump the filtrate into the crystallization tank, cool to 0°C to 10°C, stir and crystallize for 4 hours, filter in a centrifuge, diethyl ether Washed 3 times, dried, and dried under reduced pressure to obtain 1.85 kg of retigabine new crystal form D.
Embodiment 3
[0032] Add 2kg retigabine and 12L isopropyl ether into the dissolution tank, stir at room temperature until the solids are completely dissolved, filter, pump the filtrate into the crystallization tank, cool to -20°C to -10°C, stir and crystallize for 4 hours, centrifuge Machine filtration, washed 3 times with isopropyl ether, dried, and dried under reduced pressure to obtain 1.87kg retigabine new crystal form D.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com