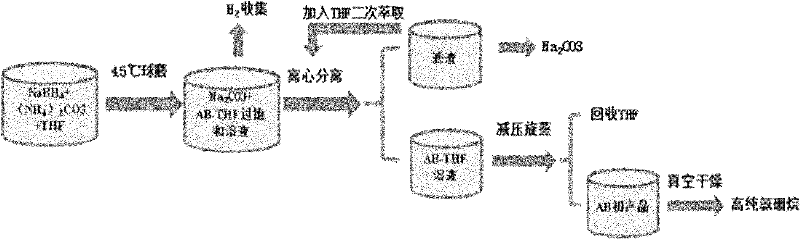
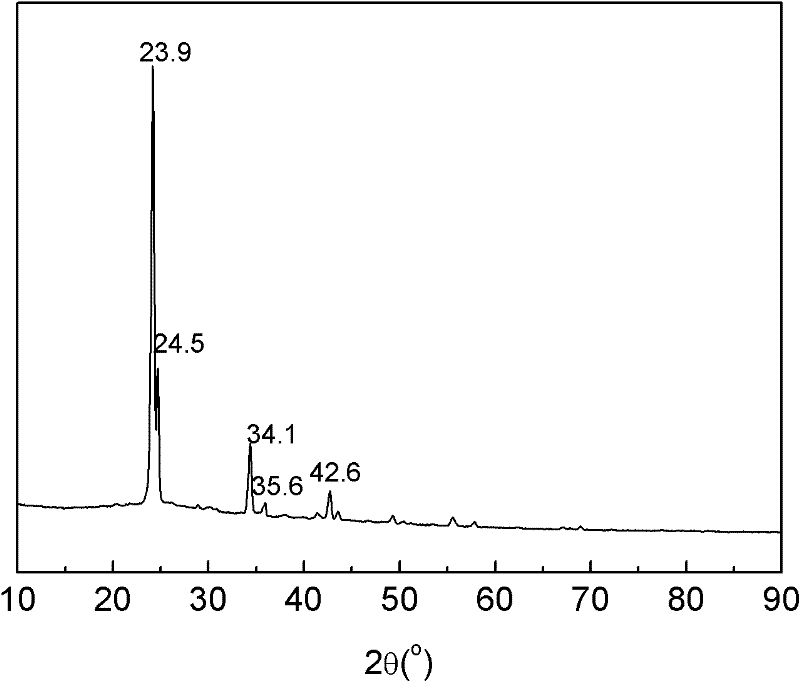
Method for preparing ammonia borane by wet chemical process
An ammonia borane and wet chemical technology, applied in the field of ammonia borane preparation, can solve the problems of reducing the particle size of ammonium salt, low reaction efficiency, slow surface erosion of ammonium salt, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 19 g of NaBH in an argon glove box 4 , 24.8 grams (NH 4 ) 2 CO 3 (Slight excess to avoid NaBH 4 excess and soluble in THF to make it difficult to separate from ammonia borane), and measure THF 500 milliliters. Put the three together into a self-designed ball mill jar (volume 2 liters). After sealing the tank body, put it into a planetary ball mill (Fischt PM-400), accurately control the temperature of the ball milling chamber at 45° C., and ball mill at 100 rpm for 45 minutes. The ball mill tank was connected with a pressure gauge, and the pressure in the tank was measured at 128psi. The amount of hydrogen calculated by the ideal gas equation was 0.5 mol, which confirmed that the reaction had been fully completed. The hydrogen in the tank was vented, and the white slurry was removed to a 600 ml centrifuge tank. After centrifugation at 3000r / min for 15min, the obtained supernatant was transferred to a 1-liter round-bottom bottle and placed in a rotary evapora...
Embodiment 2
[0018] Weigh the pre-milled NaBH in an argon glove box 4 1.9 g, (NH 4 ) 2 CO 3 2.5 grams (the ball milling condition is 200 rpm, the particle size of the sample is 100 nanometers), and 30 milliliters of THF is measured. The three were put together into a commercial autoclave (volume 100 ml). Seal the tank and connect it to a magnetic stirrer for stirring. At the same time, control the temperature of the reactor at 50°C. After 5 hours of reaction, connect the autoclave to the pressure gauge, measure the pressure in the tank at 60 psi, and calculate the amount of hydrogen with the help of the ideal gas equation. 0.0108mol, confirming that the degree of progress of the reaction is only 21.6%. It can be seen that the conventional stirring reaction is difficult to complete the preparation reaction even for the samples processed by ball milling. Although increasing the reaction temperature can further increase the reaction rate, it also brings the risk of ammonia borane produc...
Embodiment 3
[0020] Weigh 19 g NaBH4, 33.5 g (NH 4 ) 2 SO 4 (Slight excess to avoid NaBH 4 remaining and dissolved in THF), and measure 500 ml of THF. Put the three into a self-processed ball mill jar (volume 2 liters). After sealing the tank body, put it into a planetary ball mill (Fischt PM-400), accurately control the temperature of the ball milling chamber at 45° C., and ball mill at 100 rpm for 60 minutes. The ball mill tank was connected with a pressure gauge, and the pressure in the tank was measured at 128psi. The amount of hydrogen calculated by the ideal gas equation was 0.5 mol, which confirmed that the reaction had been fully completed. The hydrogen in the tank was vented, and the white slurry was removed to a 600 ml centrifuge tank. After centrifugation at 3000r / min for 15min, the obtained supernatant was transferred to a 1-liter round-bottom bottle and placed in a rotary evaporator for distillation under reduced pressure. After THF was removed, white ammonia borane crys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com