Method for preparing nanometer manganese dioxide by microwave reflux method
A technology of nano-manganese dioxide and microwave heating, applied in the direction of manganese oxide/manganese hydroxide, nanotechnology, etc., can solve the problems of high cost, difficult large-scale preparation, complicated operation, etc., and achieve low cost, controllable product quality, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
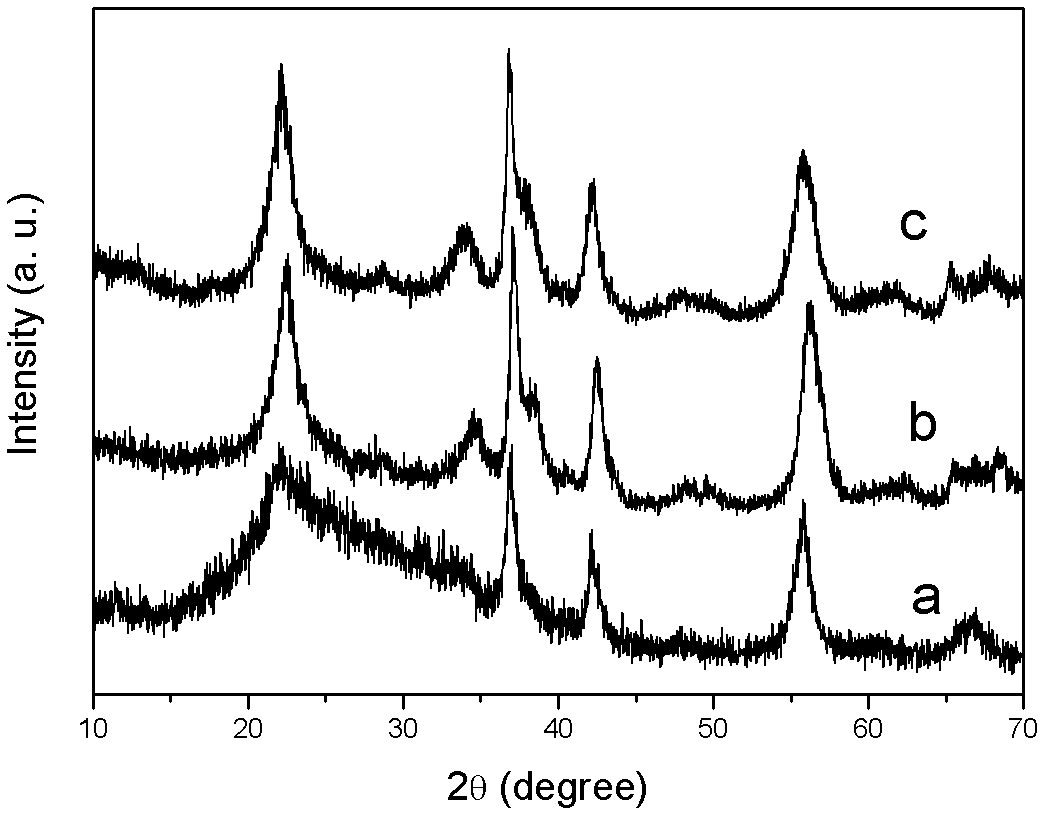
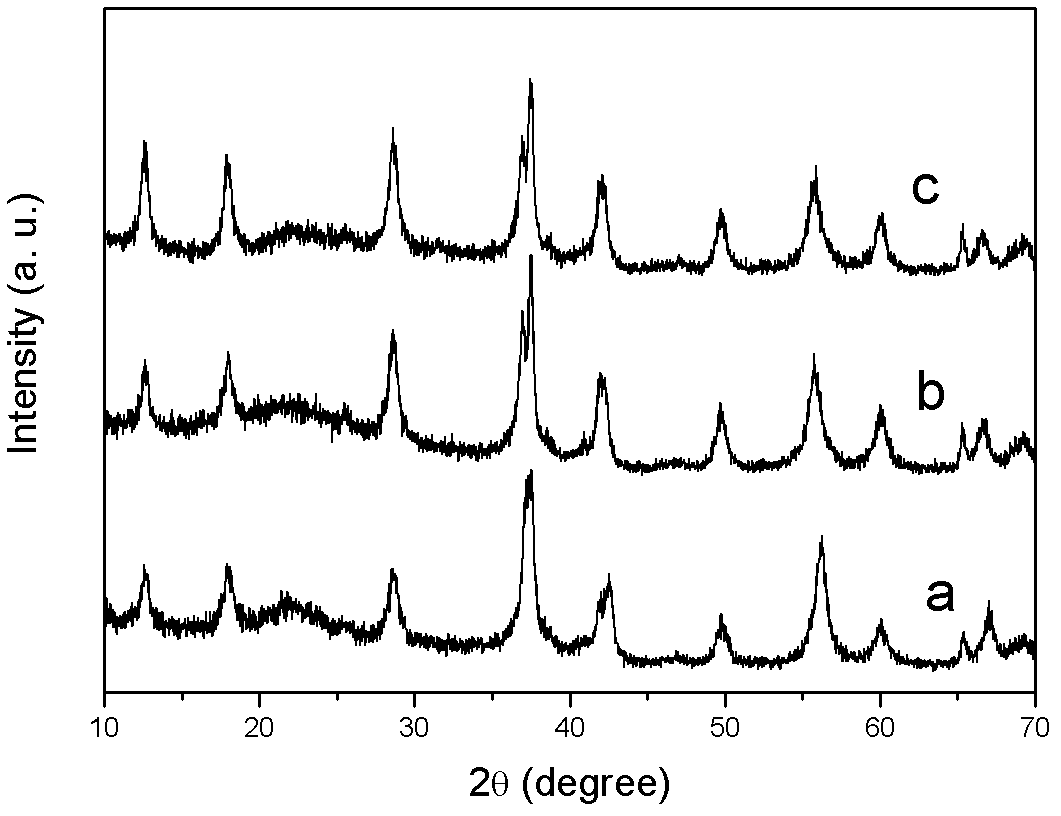
[0027] Take by weighing 2.7g potassium persulfate (analytical pure) and 1.69g manganese sulfate monohydrate (analytical pure) respectively, be dissolved in 150ml deionized water, the divalent manganese ion concentration is 0.067mol / L; The flask was then placed in the cavity of a microwave oven for heating, and the three-neck flask was connected with a condensation reflux device. The microwave power is 800W, the reaction temperature is 98°C, and the reaction time is 5min. During the reaction, a magnetic stirrer was used to stir. After the reaction, the reaction product was suction filtered, washed and dried. figure 1 and figure 2 It is the result of characterizing the product obtained in Example 1. in, figure 1 Curve a in the figure shows the XRD pattern of the product, which is very consistent with the spectral line of γ-phase manganese dioxide with structure in the JCPDS database, indicating that the product is γ-phase manganese dioxide. Such as figure 2 As shown, the...
Embodiment 2
[0029] Take by weighing 4.05g potassium persulfate (analytical pure) and 2.535g manganese sulfate monohydrate (analytical pure), be dissolved in 150ml deionized water, the divalent manganese ion concentration is 0.1mol / L; Transfer to a three-necked flask, and then place it in a microwave oven cavity for heating, and the three-necked flask is externally connected to a condensation reflux device. The microwave power is 850W, the reaction temperature is 100°C, and the reaction time is 10min. During the reaction, a magnetic stirrer was used to stir. After the reaction, the reaction product was suction filtered, washed and dried. figure 1 Curve b in the figure shows the XRD pattern of the product, which is very consistent with the spectral line of γ-phase manganese dioxide with structure in the JCPDS database, indicating that the product is γ-phase manganese dioxide.
Embodiment 3
[0031] Weigh 0.405g potassium persulfate (analytical pure) and 0.2535g manganese sulfate monohydrate (analytical pure) respectively, be dissolved in 150ml deionized water, the divalent manganese ion concentration is 0.01mol / L; Transfer to a three-necked flask, and then place it in a microwave oven cavity for heating, and the three-necked flask is externally connected to a condensation reflux device. The microwave power is 100W, the reaction temperature is 90°C, and the reaction time is 30min. During the reaction, a magnetic stirrer was used to stir. After the reaction, the reaction product was suction filtered, washed and dried. figure 1 Curve c in the figure shows the XRD pattern of the product, which is very consistent with the spectral line of γ-phase manganese dioxide with structure in the JCPDS database, indicating that the product is γ-phase manganese dioxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com