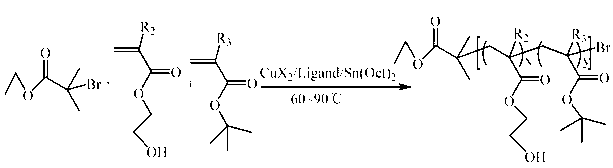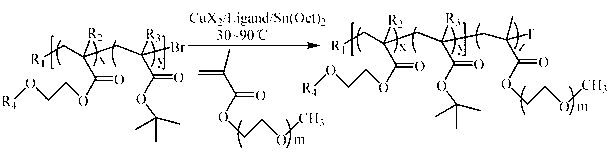PH response/hydrophobic group random copolymerization polymer, and preparation method and application thereof
A hydrophobic group and random copolymerization technology, which is applied in the direction of non-active ingredient medical preparations, pharmaceutical formulations, powder delivery, etc., can solve the problems of increasing the pH response area and incomplete drug release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
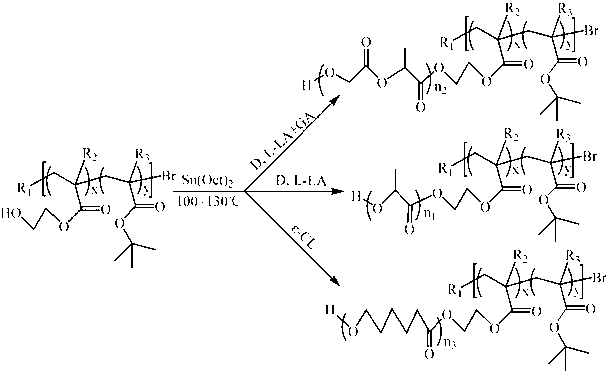
[0092] (1) Synthesis of P(HEMA- co - t BMA)-Br (A:B=14:86, A represents the terminal hydroxyl group HEMA, B represents the pH response group t BMA, the ratio is mass percentage, the same below). Take a 50 mL reaction bottle, put the stirring bar and CuBr 2 (11.2 mg) was placed in a reaction bottle, sealed and then vacuum-passed argon three times. Sequentially inject solvent toluene (20 mL), hydroxyl-terminated monomer HEMA (1.2 mL) and pH-responsive monomer t Add BMA (9.430 mL) and ligand PMDETA (104 mL) into the reaction flask, and stir for 10 min to form the catalyst complex. Then the reducing agent Sn(Oct) 2 (202.5 mg) was added to the reaction flask, stirred for 5 min, and then the initiator EBriB (147 mL) was added, and then transferred to a 70°C oil bath and stirred for 2 h. After the reaction was completed, cool to room temperature, add 50 mL of tetrahydrofuran for dilution, and then remove the catalyst by filtering through a neutral alumina column, using tetrahy...
Embodiment 2
[0117] (1) Synthesis of P(HEMA- co - t BA)-Br (A:B=11:89). Take a 50 mL reaction bottle, put the stirring bar and CuBr 2 (22.3 mg) was placed in a reaction bottle, sealed and then vacuum-passed argon three times. The solvent anisole (20 mL), monomeric HEMA (0.6 mL) and t BA (6.035 mL) and ligand bpy (312.4 mg) were added into the reaction flask, and stirred for 10 min to form the catalyst complex. Then the reducing agent Sn(Oct) 2 (405.1 mg) was added to the reaction flask, stirred for 5 min, and then the initiator EBriB (147 mL) was added, and then transferred to a 60°C oil bath and stirred for 4 h. After the reaction was completed, cool to room temperature, add 50 mL of tetrahydrofuran for dilution, and then remove the catalyst by filtering through a neutral alumina column, using tetrahydrofuran as the eluent. The obtained solution was concentrated by rotary evaporation, then slowly added to 300 mL 0 °C methanol / water (1:1 volume ratio) for precipitation, washed twice...
Embodiment 3
[0141] (1) Synthesis of P(HEMA- co - t BMA)-Br (A:B=9:91). Take a 50 mL reaction bottle, put the stirring bar and CuBr 2 (8.9 mg) was placed in a reaction bottle, sealed and then vacuum-passed argon three times. The solvent toluene (20 mL), monomeric HEMA (1.2 mL) and t Add BMA (15.1 mL) and ligand PMDETA (83.5 mL) into the reaction flask, and stir for 10 min to form the catalyst complex. Then the reducing agent Sn(Oct) 2 (162 mg) was added to the reaction flask, stirred for 5 min, and then the initiator EBriB (147 mL) was added, then transferred to an 80°C oil bath and stirred for 1 h. After the reaction was completed, cool to room temperature, add 50 mL of tetrahydrofuran for dilution, and then remove the catalyst by filtering through a neutral alumina column, using tetrahydrofuran as the eluent. The obtained solution was concentrated by rotary evaporation, then slowly added to 300 mL 0 °C methanol / water (1:1 volume ratio) for precipitation, washed twice with deionize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical micelle concentration (mass) | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com