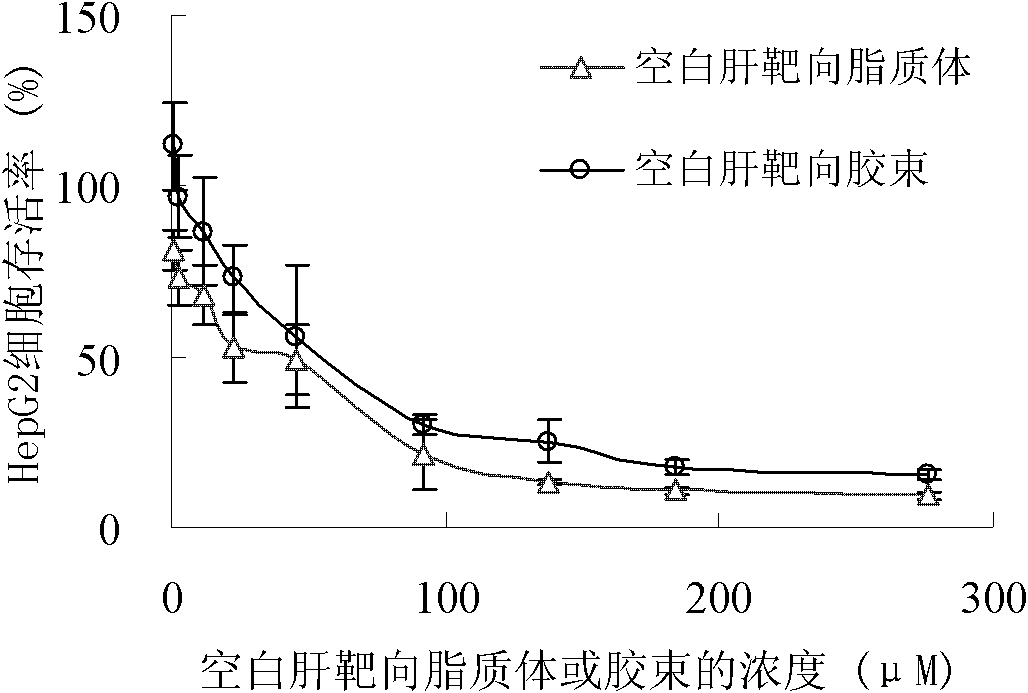
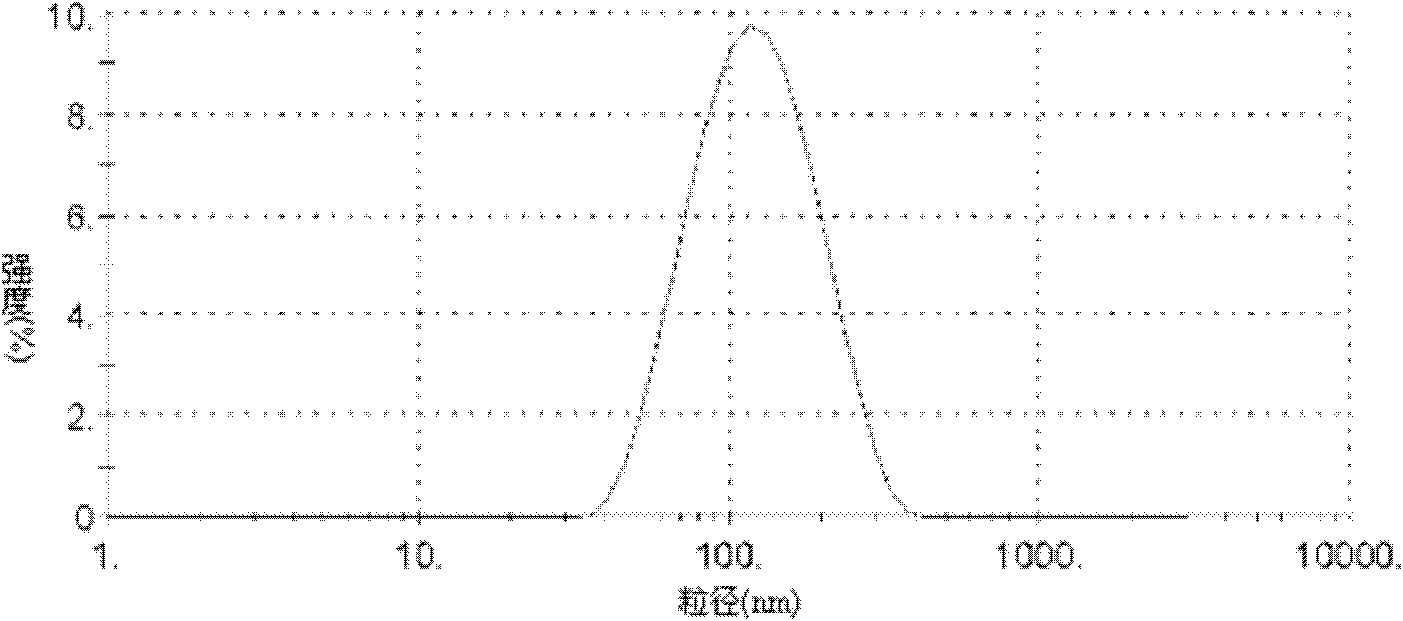
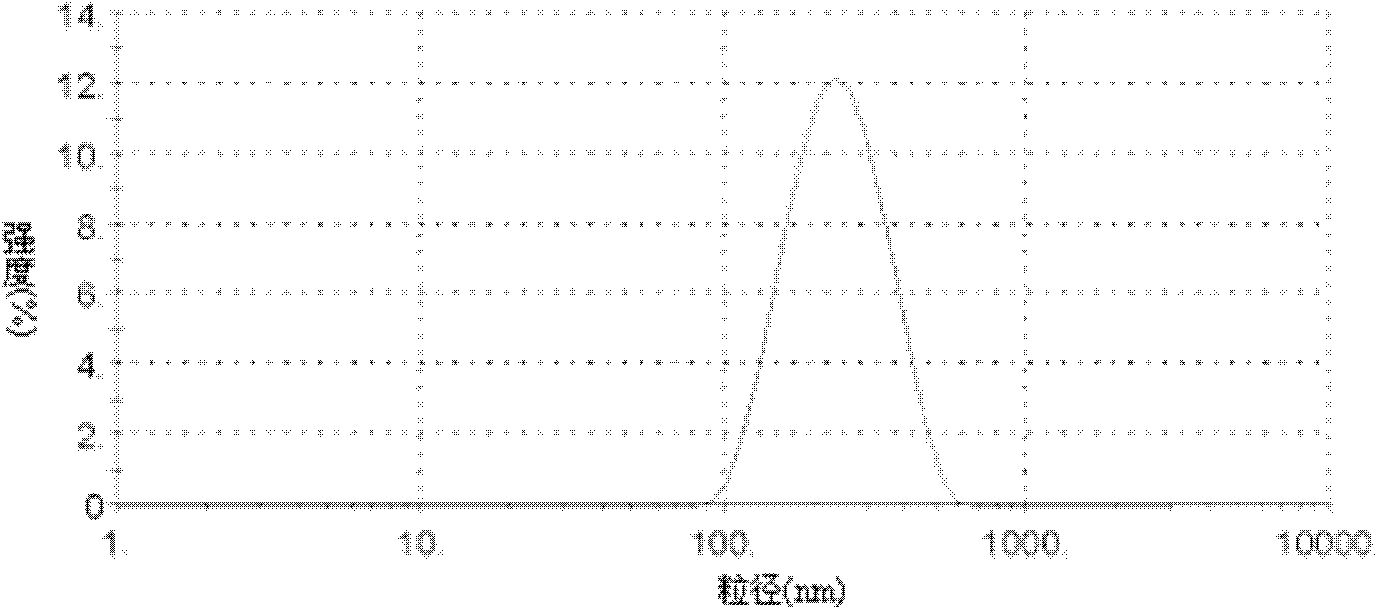
Glycyrrhetinic acid-modified lipid, liver targeting liposome, micelle and compound, and their preparation method
A glycyrrhetic acid, liver targeting technology, applied in the field of medicine, can solve the problems of increasing the incorporation ratio, phagocytosis, and difficulty in ensuring biocompatibility, and achieves the effect of increasing the enrichment concentration and improving the targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 glycyrrhetinic acid modified cholesterol:
[0055] Weigh 5g of glycyrrhetinic acid and place it in a 500mL round bottom flask, add 250mL of dichloromethane into the bottle, and magnetically stir to dissolve. Then add 2.5g of EDC into the bottle, stir magnetically until dissolved, then add 4g of cholesterol, continue to stir, and react at room temperature for 12 hours. The organic layer was washed with 1 mol / L HCl solution, saturated sodium bicarbonate solution and saturated NaCl solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the organic solvent. The obtained solid was subjected to column chromatography (petroleum ether: acetone = 90:10-30:70 gradient elution) to obtain 7.4 g of a light yellow solid (yield 82.6%).
[0056] Above-mentioned solid also can adopt high performance liquid phase to purify, and its chromatographic condition is methanol / acetonitrile-water system, and detector is ul...
Embodiment 2
[0057] The preparation of embodiment 2 glycyrrhetinic acid modified phospholipids:
[0058] Weigh 1g of glycyrrhetinic acid and place it in a 250mL round bottom flask, add 100mL of dichloromethane into the bottle, and magnetically stir to dissolve. Then add 0.5g EDC to the bottle, stir magnetically until dissolved, cool in an ice-water bath, add 0.4g distearoylphosphatidylethanolamine (hereinafter referred to as DSPE), continue to stir until completely dissolved, keep ice-water bath cooling, avoid light for 24 hours . The organic layer was washed with 1 mol / L HCl solution, saturated sodium bicarbonate solution and saturated NaCl solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the organic solvent. The resulting solid was subjected to column chromatography (n-hexane:acetone=95:5-25:75 gradient elution) to obtain 0.5 g of a white solid (yield 76.8%).
[0059] Above-mentioned solid also can adopt high performance liquid phase to p...
Embodiment 3
[0060] Example 3 Preparation of Glycyrrhetinic Acid Modified PEGylated Cholesterol:
[0061] Weigh 5g of glycyrrhetinic acid and place it in a 500mL round bottom flask, add 250mL of dichloromethane into the bottle, and magnetically stir to dissolve. Then add 2.5g of EDC and 0.9g of 4-dimethylaminopyridine (hereinafter referred to as DMAP) into the bottle, stir magnetically until dissolved, then add 18g of polyethylene glycol 2000, continue to stir, and react at room temperature for 12 hours. The organic layer was washed with 1 mol / L HCl solution, saturated sodium bicarbonate solution and saturated NaCl solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the organic solvent. The resulting solid was subjected to column chromatography (dichloromethane:methanol=0:100-30:70 gradient elution) to obtain 7.8 g of a light yellow solid. The solid was placed in a 500 mL round bottom flask, 200 mL of dichloromethane was added into the bottle, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com