Promethazine hydrochloride microenema prescription and preparation method thereof
A technology of promethazine hydrochloride and enema, applied in the directions of anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of great difference in relative bioavailability, insufficient flexibility of suppositories, easy oxidation and discoloration, and the like, Achieving good rectal absorption properties, good clinical applicability and convenience, and easy adjustment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
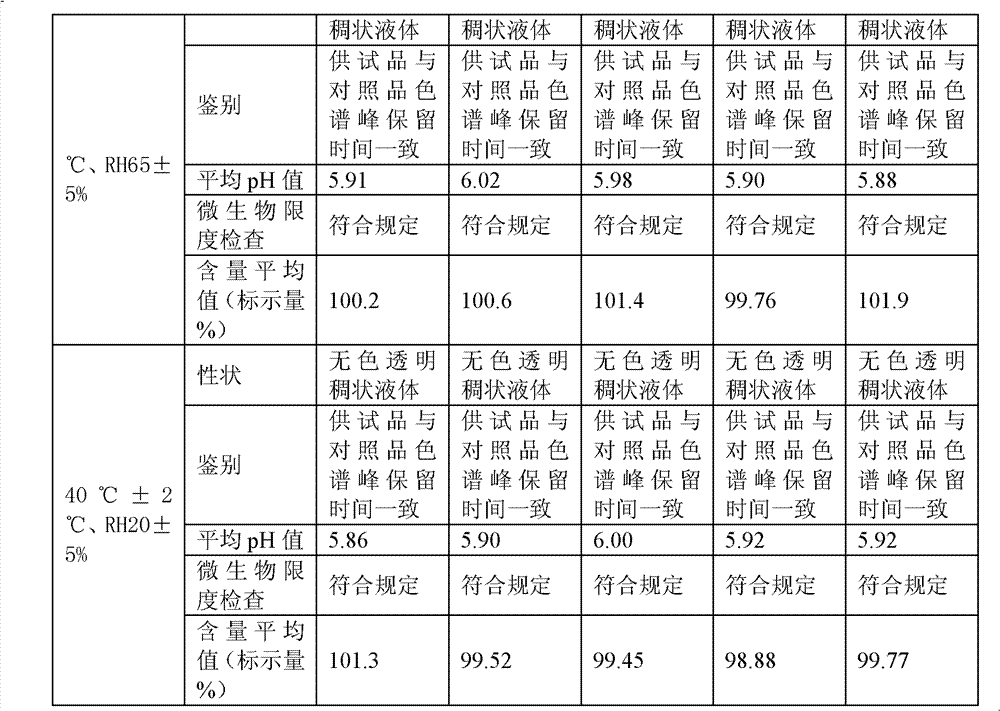
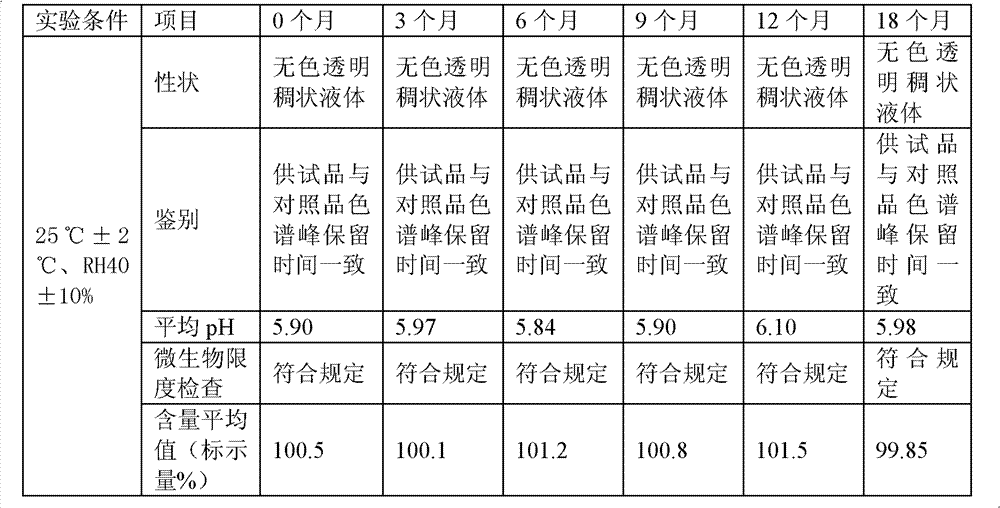
Image
Examples
Embodiment 1
[0017] Promethazine hydrochloride micro-enema prescription of the present invention comprises the following components by weight: hydroxypropyl methylcellulose: 5g, sodium bisulfite: 1g, sodium sulfite: 0.6g, vitamin C: 0.5g, EDTA- 2Na: 0.1 g, promethazine hydrochloride: 2.5 g, and ethylparaben: 0.5 g.
[0018] The preparation method of the promethazine hydrochloride miniature enema prescription of the present embodiment may further comprise the steps:
[0019] a. Dissolve 5 g of hydroxypropyl methylcellulose in 400 ml of purified water to form liquid A;
[0020] b. Dissolve 1g of sodium bisulfite, 0.6g of sodium sulfite, 0.5g of vitamin C and 0.1g of EDTA-2Na in 400ml of purified water, then add 2.5g of promethazine hydrochloride to dissolve to form liquid B;
[0021] c. Ethylparaben 0.5 is dissolved in 100ml boiling water to form liquid C;
[0022] d. Add liquid C to liquid B and mix evenly to form liquid D;
[0023] e. Add liquid D into liquid A and stir evenly, add puri...
Embodiment 2
[0026] Promethazine hydrochloride miniature enema prescription of the present invention comprises the following components by weight: hydroxypropyl methylcellulose: 10g, sodium bisulfite: 1g, sodium sulfite: 0.3g, vitamin C: 0.1g, EDTA- 2Na: 0.3 g, promethazine hydrochloride: 2.5 g, and ethylparaben: 0.5 g.
[0027] The preparation method of the promethazine hydrochloride miniature enema prescription of the present embodiment may further comprise the steps:
[0028] a. Dissolve 10 g of hydroxypropyl methylcellulose in 400 ml of purified water to form liquid A;
[0029] b. Dissolve 1g of sodium bisulfite, 0.3g of sodium sulfite, 0.1g of vitamin C and 0.3g of EDTA-2Na in 400ml of purified water, then add 2.5g of promethazine hydrochloride to dissolve to form liquid B;
[0030] c. Dissolve 0.5 g of ethylparaben in 100 ml of boiling water to form liquid C;
[0031] d. Add liquid C to liquid B and mix evenly to form liquid D;
[0032] e. Add liquid D into liquid A and stir evenl...
Embodiment 3
[0035] Hydroxypropyl methylcellulose: 7g, sodium bisulfite: 1g, sodium sulfite: 0.45g, vitamin C: 0.3g, EDTA-2Na: 0.2g, promethazine hydrochloride: 2.5g and ethylparaben: 0.5 g.
[0036] The preparation method of the promethazine hydrochloride miniature enema prescription of the present embodiment may further comprise the steps:
[0037] a. Dissolve 7 g of hydroxypropyl methylcellulose in 400 ml of purified water to form liquid A;
[0038]b. Dissolve 1g of sodium bisulfite, 0.45g of sodium sulfite, 0.3g of vitamin C and 0.2g of EDTA-2Na in 400ml of purified water, then add 5g of promethazine hydrochloride to dissolve to form liquid B;
[0039] c. Dissolve 0.5 g of ethylparaben in 100 ml of boiling water to form liquid C;
[0040] d. Add liquid C to liquid B and mix evenly to form liquid D;
[0041] e. Add liquid D to liquid A and stir evenly, add purified water to 1000ml, stir evenly again, divide into 10ml glass bottles under 100,000-grade purification conditions, and obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com