Heavy targeted modification method for herpes simplex viruses and application thereof
A herpes simplex virus, targeted modification technology, applied in the field of medicine and biology, can solve the problems of unusable tumor patients and large clinical limitations, and achieve the effects of improving safety, avoiding immune response, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Cultivation and purification of HSV-1
[0043] Vero cells were inoculated in a cell culture dish with a diameter of 12.5 cm. When the cells grew to 80% full, the cell culture medium was replaced with DMEM medium containing 5% newborn calf serum, and the virus HSV was inoculated with MOI=0.05. at 37°C with 5% CO 2 Continue culturing for 72 hours in the incubator. The cell culture supernatant and cell debris were collected into sterile cryopreservation tubes and stored at -80°C.
[0044] The virus supernatant and cells collected above were repeatedly frozen and thawed three times, so as to fully lyse the cells and release the HSV virus particles in the cells. Put the virus liquid that had been frozen and thawed three times into a centrifuge tube, centrifuge at 5000 rpm at 4°C for 10 minutes, and collect the virus supernatant. Centrifuge at 12000 rpm at 4°C for 30 minutes to further remove cell debris in the virus supernatant. Add 25 ml of the preliminarily purified vi...
Embodiment 2
[0046] Synthesis and Characterization of FA-PEG
[0047] Folic acid and NHS form activated ester, that is, folic acid is dissolved in dimethyl sulfoxide (DMSO), reacted with dicyclohexylcarbodiimide (DCC), n-hydroxysuccinamide at a molar ratio of 1:2:2 , stirred for 18 hours, and the whole reaction was carried out under nitrogen protection. The resulting suspension was centrifuged at 15,000 rpm for 30 minutes to remove white insoluble matter and obtain a yellow supernatant.
[0048] NH with a molar ratio of 1:8 to the folic acid activator 2 -PEG-COOH was dissolved in DMSO, reacted with folic acid activator under the condition of nitrogen protection for 4 hours, dialyzed the obtained substance in DMSO and ultrapure water at 4°C for 3 days and 2 days respectively, and the final product was in Freeze-dry at -20°C.
[0049] Take a part of the FA-PEG freeze-dried product (about 5 mg) and dissolve it in deuterated DMSO, and detect it by nuclear magnetic resonance to confirm the r...
Embodiment 3
[0051] FA-PEGylation of herpes simplex virus
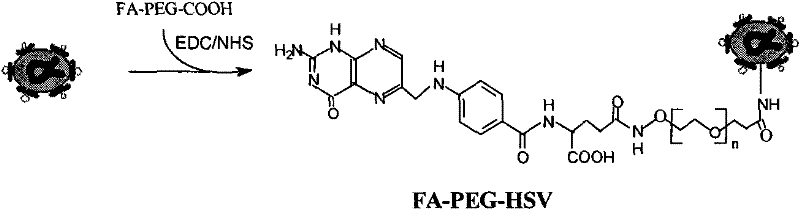
[0052] Dissolve methoxy-PEG-NHS (mPEG) in 2ml HBSS, add HSV (the ratio of mPEG to HSV is 1PFU, add 1×10 7 mPEG molecules), reacted in ice bath for 4 hours. The PEG-FA obtained after the reaction was dialyzed in ultrapure water at 4°C for 2 days, and the final product was stored at -80°C.
[0053] Dissolve FA-PEG-COOH in 2ml HBSS, add EDC and NHS for activation, the molar ratio is 1:10:10, react at room temperature for 1 hour, take the activated FA-PEG-COOH and dissolve it in 2ml HBSS, add HSV (The ratio of HSV to FA-PEG-COOH is 1PFU of HSV adding 1×10 7 FA-PEG-COOH molecules), reacted in ice bath for 4 hours. The PEG-FA obtained after the reaction was dialyzed in ultrapure water at 4°C for 2 days, and the final product FA-PEG-HSV was stored at -80°C.
[0054] The purity of FA-PEG-HSV was further detected, specifically: the fluorescein ammonia dye was dissolved in acetone to prepare a 7 mg / mL fluoresceinamide solution. Add 900...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Luminous wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com