Fusarium head blight virus toxin degrading gene and expression thereof
A viral toxin and gene technology, applied in the field of biomedicine, can solve problems such as the absence of Fusarium graminearum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
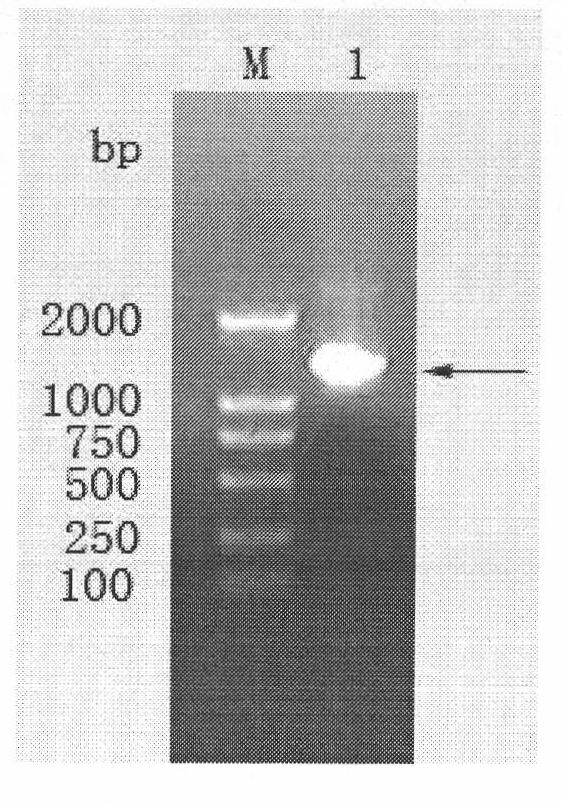
[0028]Example 1 Expression of Fusarium graminearum degradative toxin gene Tri101
[0029] 1. Extraction of total RNA and synthesis of cDNA
[0030] Inoculate Fg0623 spores cultured on fresh PDA slant into liquid medium, shake at 150rpm, 28°C for 48h; draw 1mL of the cultured bacteria into a 1.5mL sterile EP tube, and centrifuge at 10000rpm for 10min; collect the bacteria, according to RNAiso TM The total RNA of the bacteria was extracted according to the method in the Plus manual. Using the extracted total RNA as a template, cDNA was synthesized. RT1 system: RNase Free dH 2 O 6.5 μL, dNTP-Mix (10 mM) 1 μL, Oligo dT (50 μM) 2 μL, Total RNA 0.5 μL were reacted on a PCR machine at 65°C for 5 min, and cooled on ice. RT2 system: 10 μL of the above denatured and annealed reaction solution, 5×PrimeScript TM Buffer 4μL, RNase Inhibitor (40U / μL) 0.5μL, PrimeScript TM RTase (200U / μL) 1μL, RNase Free dH 2 O 4.5 μL. Reaction program: 30°C for 10 minutes, 42°C for 60 minutes, 70...
Embodiment 2
[0078] Example 2 Sequence Analysis of Fusarium graminearum Degradative Toxin Gene Tri101
[0079] The positive clone samples successfully identified by enzyme digestion were sent to Sangon Company for DNA sequence determination. Using the DNAclub software and the BLAST function of the NCBI website, the determined sequences were compared and analyzed.
[0080]The result of sequence determination of Fg0623Tri101 gene showed that the Fg0623Tri101 gene contained a complete open reading frame with a total length of 1356bp. The gene has been registered in GenBank with accession number GQ907236. It is related to the nucleotide sequence of Fusarium graminearum Tri101 gene reported by Kimura (Kimura M, Shingu Y, Yoneyama K, et al. Features of Tri101, the trichothecene 3-O-acetyl-transferase gene, related to the self-defense mechanism in Fusarium graminearum[J]. Biosci Biotechnol Biochem, 1998, 62(5): 1033-1036.) has the highest homology, 99.78%, and the base sequence only changes fro...
Embodiment 3
[0081] Embodiment 3 Tri101 Gene Coded Amino Acid Sequence Analysis
[0082] The protein analysis software provided by the website http: / / www.expasy.org / tools / #translate analyzed the basic characteristics of the amino acid sequence encoded by the Tri101 gene, and the results showed that the molecular weight of the protein predicted by the gene was 49.45kDa, and the isoelectric point In the amino acid composition, there are 47 positively charged amino acids (Arg, Lys), accounting for 10.42%, 57 negatively charged amino acids (Asp, Glu), accounting for 12.64%, 217 hydrophobic amino acids, accounting for 48.12%, and hydrophilic 184 sex amino acids, accounting for 40.80%, see Table 1 and image 3 .
[0083] Table 1 Amino acid content of Tri101 protein
[0084]
[0085] Note: *hydrophobic amino acid, #hydrophilic amino acid
[0086] The protein encoded by this gene is trichothecene 3-O-acetyltransferase, and the results of Blast database analysis show that it belongs to the tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com