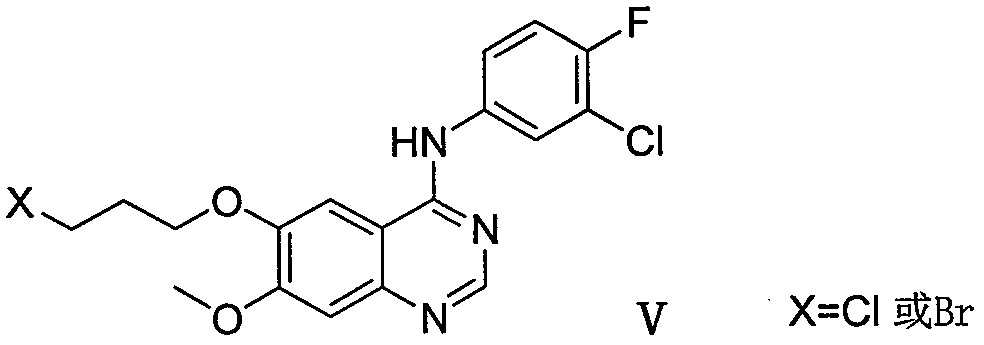
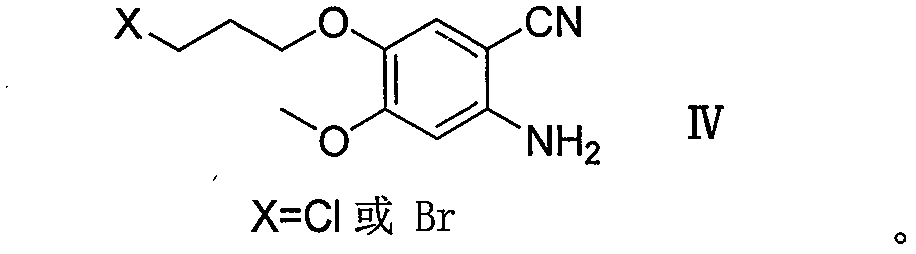
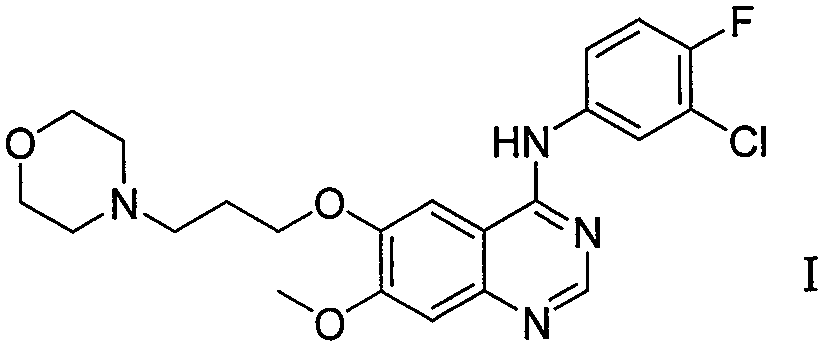
Method for preparing gefitinib and intermediate thereof
A gefitinib, Chinese-style technology, applied in the field of preparation of gefitinib and its intermediates, can solve the problem of low total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of 4-methoxy-3-(3-chloropropoxy) benzonitrile
[0069] Take 20.10g (134mmol) of 3-hydroxy-4-methoxybenzonitrile, 16.0mL (161mmol) of 1,3-bromochloropropane, anhydrous K 2 CO 3 18.52g (134mmol), 5.00g of polyethylene glycol-400 and 150mL of absolute ethanol were placed in a 250mL three-necked bottle, and heated to reflux for 5h. Filter off K while hot 2 CO 3 , The filtrate was concentrated to dryness under reduced pressure, and the obtained solid was dissolved in 250 mL of methyl tert-butyl ether, and washed 3 times with water in a separatory funnel, each time with 50 mL of water. Anhydrous Na for organic layer 2 SO 4 Dry and concentrate to dryness to obtain 27.31 g (121 mmol) of 4-methoxy-3-(3-chloropropoxy)benzonitrile as a white solid, with a yield of 90%.
[0070] mp: 73.1~74.1℃; IR v max (KBr)cm -1 : 3008, 2968, 2219, 1598, 1581, 1517, 1422, 1270, 1246, 1139, 1017, 860, 810, 653, 617; 1 H-NMR (300MHz, CDCl 3 )δ: 2.30(m, 2H), ...
Embodiment 2
[0071] Embodiment 2: Preparation of 2-nitro-4-methoxy-5-(3-chloropropoxy)benzonitrile
[0072] Take 10.00 g (44.4 mmol) of 4-methoxy-3-(3-chloropropoxy) benzonitrile and dissolve it in 25 mL of acetic acid, mix and cool 25 mL of sulfuric acid (70%) and 5 mL of nitric acid (70%) Finally, it was added dropwise to the system under an ice-water bath, and after the dropwise addition was completed, the reaction was continued at 25°C for 50h. Then 200 mL of water was added to the system, and a milky yellow solid was precipitated, which was suction filtered, and the filter cake was washed with water until neutral, and dried to obtain a light yellow solid 2-nitro-4-methoxy-5-(3-chloropropoxy ) Benzonitrile 11.48g (42.5mmol), yield 96%.
[0073] mp: 111.8~112.2℃; IR v max (KBr)cm -1 : 3064, 2945, 2225, 1570, 1527, 1337, 1293, 1234, 1059, 1004, 938, 887, 801; 1 H-NMR (300MHz, CDCl 3 )δ: 2.35(m, 2H), 3.78(t, J=5.93Hz, 2H), 4.01(s, 3H), 4.30(t, J=5.61Hz, 2H), 7.24(s, 1H), 7.80( s, 1H...
Embodiment 3
[0074]Embodiment 3: Preparation of 2-amino-4-methoxy-5-(3-chloropropoxy)benzonitrile
[0075] Take 13.53 g (50.1 mmol) of 2-nitro-4-methoxy-5-(3-chloropropoxy) benzonitrile, a mixture of methanol and water (methanol: water = 3: 1) 150 mL, Na 2 S 2 o 4 26.12g (150.0mmol) was placed in a 250mL two-neck flask, heated to 50°C for 2.5h. Then the temperature of the reactant was raised to 70°C, and 50 mL of 25% dilute hydrochloric acid was added in portions, and the addition was completed within 0.5 h, and cooled to room temperature. Adjust the pH value of the mixture to about 10 with 50% NaOH aqueous solution, filter with suction, wash the filter cake with water, and dry to obtain a light yellow solid 2-amino-4-methoxy-5-(3-chloropropoxy)benzene Formaldehyde 11.29g (47.0mmol), yield 94%.
[0076] mp: 74.6~75.0℃; IR v max (KBr)cm -1 : 3464, 3359, 3234, 3072, 2930, 2201, 1640, 1619, 1576, 1524, 1507, 1270, 1236, 1130, 1008, 948, 867, 827; 1 H-NMR (300MHz, DMSO-d 6 )δ: 2.08(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com