A long-acting injection preparation of steroidal 5α-reductase inhibitor and its preparation method
A technology of reductase inhibitor and long-acting injection preparation, which is applied in the field of long-acting injection preparation and its preparation, can solve the problems of inconvenience and no patent and literature report of steroid 5α-reductase inhibitor long-acting injection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
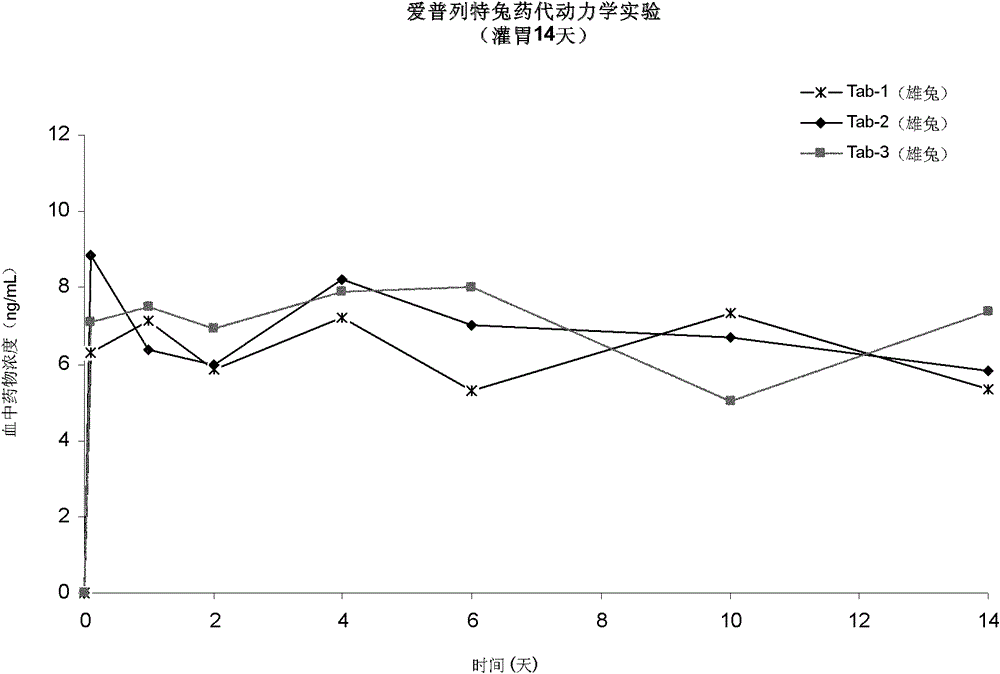
[0052] Will 4g RG503 was dissolved in 9 g of dimethyl sulfoxide (DMSO) to obtain a viscous polymer solution. Add 1.2g of Apretide, mix evenly, sterilize with cobalt radiation, and aseptically dispense, each syringe is pre-filled with 1.4g of finished product.
[0053] In vitro drug release checks:
[0054] Take 8 finished products, inject the same amount of the finished product in each syringe into the bottom of two 1L reagent bottles respectively, record the injection volume, slowly add 900 mL of 0.001 mol preheated at 37 °C along the wall of the reagent bottle / L sodium hydroxide solution, put it in a constant temperature shaking box at 37°C and shake at a speed of 100rpm, and take two bottles of samples at 0.5 hours, 2 hours, 24 hours, 2 days, 4 days, 6 days, 10 days, and 14 days for testing . Absorbance was measured at 267 nm by UV spectrophotometry. Calculate the release amount by external standard method, and draw the release curve as figure 1 shown, from figure 1...
Embodiment 2
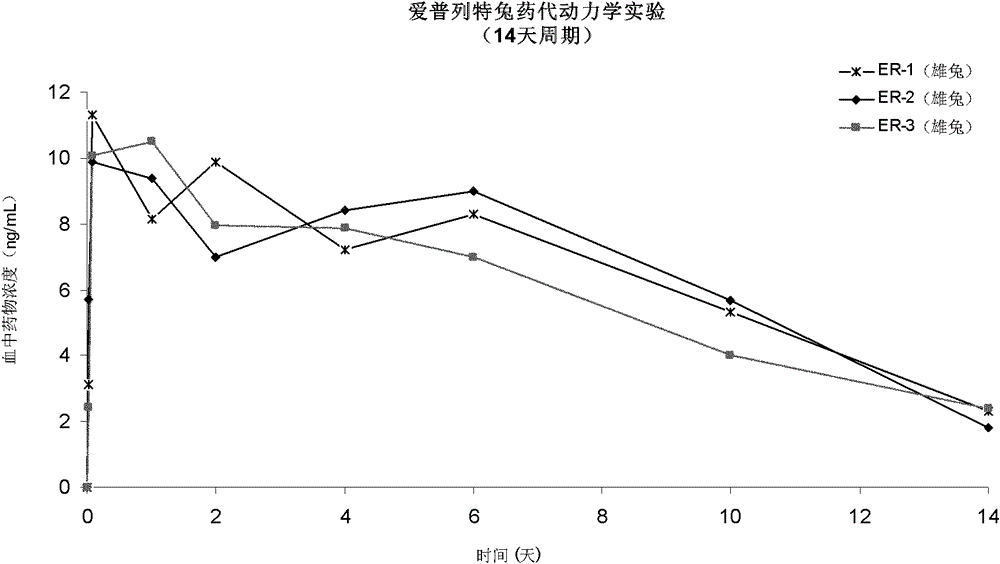
[0058] 3.5g RG752H was dissolved in 5.2 g of ethyl acetate to obtain a viscous polymer solution. After adding 0.45g of dutasteride and 10mg of zinc carbonate and suspending evenly, each syringe was prefilled with 0.9mL of the suspension and freeze-dried. Resuspend with sterile N-methylpyrrolidone before injection.
[0059] In vitro drug release checks:
[0060] Take 5 tubes of the finished product, resuspend them with sterile N-methylpyrrolidone, inject the finished product in the syringe into the bottom of 4 reagent bottles with a volume of 1L in 4 times, record the injection volume, and slowly add it along the wall of the reagent bottle 900mL phosphate buffer solution containing 2% SDS, pH 7.0 preheated at 37°C, shaken at 100rpm in a constant temperature shaking box at 37°C, and shaken at 0.5 hour, 1 day, 2 days, 4 days, 7 days, respectively. Two bottles of samples were taken at 14 days, 21 days, 28 days, 35 days and 42 days for testing. Absorbance was measured at 250 n...
Embodiment 3
[0064] Pick R503H and After mixing R203H at a weight ratio of 8:2, dissolve 4g of the mixture in 9g of ethanol, and suspend 1.2g of micronized aprelate (75μm<D90<100μm), 25mg of magnesium hydroxide, 0.50 g of sucrose acetate isobutyrate (SAIB). Prepare a normal saline solution containing 2.0% medium chain triglycerides. Take 10 g of the above-mentioned physiological saline solution and add it into the suspension, high-speed emulsification, freeze-drying, and granulation. Make the particle diameter less than 100μm. Divide according to dosage requirements. Resuspend with 2.5% sterile phospholipid solution before injection.
[0065] In vitro drug release checks:
[0066]Into 16 bottles of 900mL 0.001mol / L sodium hydroxide solution preheated at 37°C, weigh 750mg of the above particles respectively, and shake at a speed of 100rpm in a constant temperature shaking box at 37°C. Two bottles of samples were taken each day, 4 days, 6 days, 10 days, and 14 days for testing. Abs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com