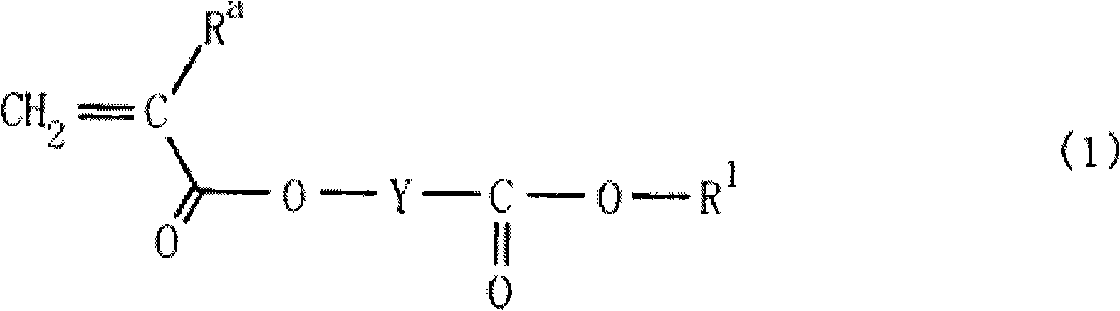
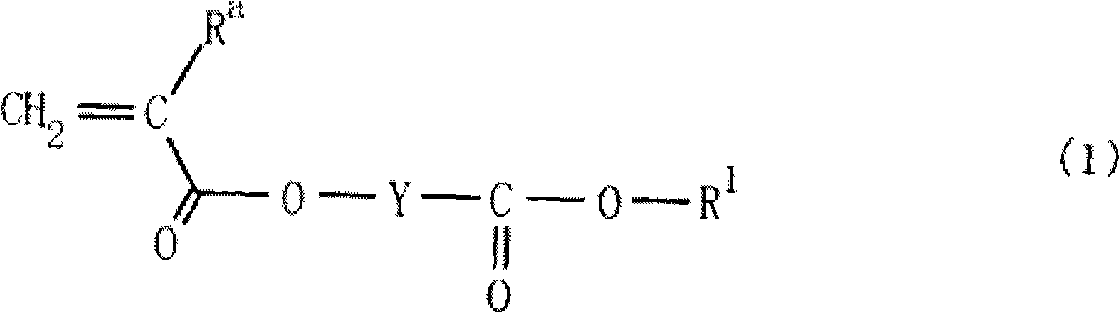
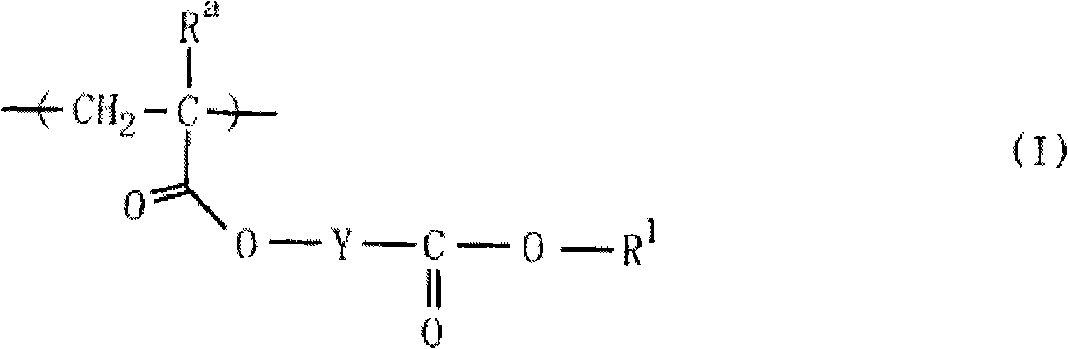
Monomer having lactone skeleton, polymer compound and photoresist composition
A polymer compound and skeleton technology, applied in the field of photoresist monomers, polymer compounds and photoresist compositions, can solve problems such as poor solubility, achieve improved solubility, excellent solubility, The effect of sharp patterns
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0117] 2-Methacryloyloxyacetoxy-3,3-dimethyl-γ-butyrolactone was produced according to the following reaction procedure formula.
[0118] [chemical formula 9]
[0119]
[0120] Add 10.0 g (0.0768 mol) of 2-hydroxy-3,3-dimethyl-γ-butyrolactone represented by formula (5a) and 20.0 g of acetonitrile in the three-necked flask and dissolve it, then add 1,8-di Azabicyclo[5.4.0]undec-7-ene (DBU) 38.6g (0.2536mol), and the internal temperature was raised to 30°C. After that, 26.0 g (0.2305 mol) of chloroacetyl chloride represented by the formula (4a) was slowly added dropwise at an internal temperature of 45° C. or lower in a nitrogen atmosphere, and then stirred at 40° C. for 5 hours. Then, after adding and stirring the reaction liquid to the liquid mixture of 50 g of ethyl acetate and 50 g of pure water, it liquid-separated and extracted the organic layer. After the extracted organic layer was washed 3 times with 36 g of 8% by weight aqueous sodium bicarbonate solution, 2 times...
Embodiment 2
[0127] Synthesis of polymer compounds with the following structures
[0128] [chemical formula 10]
[0129]
[0130] In a round-bottomed flask equipped with a reflux tube, a stirring bar, and a three-way cock, under a nitrogen atmosphere, add 41.65 g of propylene glycol monomethyl ether acetate (PGMEA) and 17.85 g of propylene glycol monomethyl ether (PGME), and keep the temperature While stirring at 80°C, the following monomer solution consisting of 2-methacryloyloxyacetoxy-3,3-dimethyl- γ-butyrolactone 12.07g (47.1mmol), 1-hydroxy-3-methacryloyloxyadamantane 5.57g (23.5mmol), 1-(1-methacryloyloxy-1-methyl ethyl Base) 12.36 g (47.1 mmol) of adamantane, 1.80 g of dimethyl 2,2'-azobisisobutyrate [manufactured by Wako Pure Chemical Industries, Ltd., trade name "V-601"], 77.35 g of PGMEA And PGME 33.15g mixed. After the dropwise addition was completed, stirring was continued for 2 hours. After the polymerization reaction finished, this reaction solution was added dropwise ...
Embodiment 3
[0132] Synthesis of polymer compounds with the following structures
[0133] [chemical formula 11]
[0134]
[0135] Except that the monomer components used in Example 2 were changed to the following components, the same operation as in Example 2 was carried out to obtain 25.2 g of the target resin. The monomer components are: 2-methacryloyloxy Acetoxy-3,3-dimethyl-γ-butyrolactone 12.63g (49.3mmol), 1-hydroxy-3-methacryloyloxyadamantane 5.82g (24.6mmol), 2-methylpropene 11.55 g (49.3 mmol) of acyloxy-2-methyladamantane. GPC analysis was carried out on the recovered polymer, and the results showed that its Mw (weight average molecular weight) was 8900, and its molecular weight distribution (Mw / Mn) was 1.89.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com