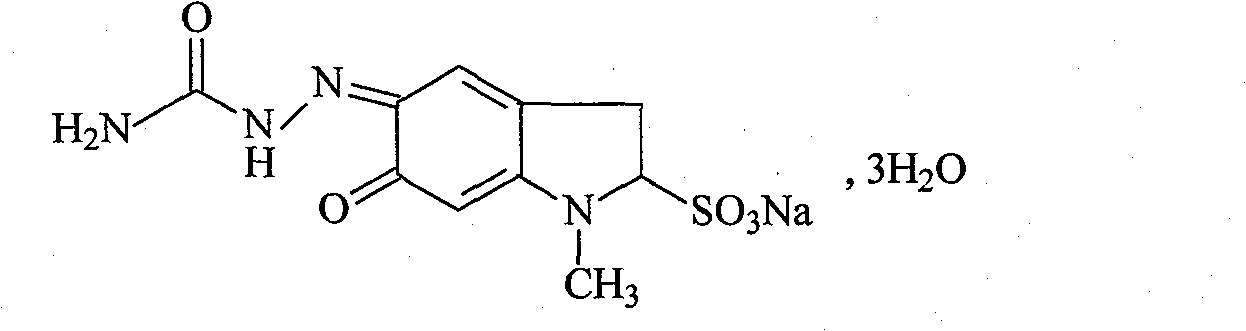
Carbazochrome sodium sulfonate freeze-dried powder injection and preparation method thereof
A technology of sodium carbosulfonate and freeze-dried powder injection, applied in the field of sodium carbosulfonate freeze-dried powder injection and its preparation, to achieve the effect of eliminating the risk of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Sodium carbosulfonate 20g
[0048] Mannitol 100g
[0049] Citric acid-sodium citrate buffer 100ml
[0050] Add water for injection to 1000ml
[0051] Made 1000 pieces
[0052] Preparation process: Weigh the prescribed amount of carsulfone sodium, add the prescribed amount of water for injection, stir and dissolve at 50°C, then weigh the prescribed amount of mannitol, stir and dissolve, add the prescribed amount of citric acid-sodium citrate buffer Then add activated carbon in the prescribed amount, stir for 30 minutes, first filter for decarburization, then filter with a 0.22um filter membrane, detect the intermediate, fill, freeze-dry, add a cover, test, and pack to obtain the frozen sodium carbosulfonate Finished dry powder injection.
Embodiment 2
[0054] Sodium carbosulfate 40g
[0055] Mannitol 200g
[0056] Sodium dihydrogen phosphate 5.5g
[0057] Sodium hydroxide test solution
[0058]Add water for injection to 2000ml
[0059] Made 1000 pieces
[0060] Preparation process: a. Weigh the prescribed amount of sodium carsulfone, add an appropriate amount of water for injection, stir and dissolve at 50°C, then add the prescribed amount of mannitol, stir and dissolve; b, add the prescribed amount of sodium dihydrogen phosphate with appropriate amount of water for injection Dissolve, adjust the pH value of the sodium hydroxide test solution to 5.0, add it to the solution a, then add water to the full amount, then add 0.1% activated carbon, stir for 30 minutes, first filter for decarburization, and then filter with a 0.22um filter membrane to detect the middle body, filling, freeze-drying and capping, inspection, and packaging to obtain the finished carbosulfonate freeze-dried powder injection.
Embodiment 3
[0062] Sodium carbosulfonate 20g
[0063] Mannitol 100g
[0064] Citric acid-sodium citrate buffer 50ml
[0065] Phosphate buffer 50ml
[0066] Add water for injection to 1000ml
[0067] Preparation process: Weigh the prescribed amount of carsulfone sodium, add the prescribed amount of water for injection, stir and dissolve at 50°C, then weigh the prescribed amount of mannitol, stir and dissolve, add the prescribed amount of citric acid-sodium citrate buffer solution and phosphate buffer solution, then add 0.1% activated carbon, stir for 30 minutes, first filter for decarburization, and then use 0.22um filter membrane to filter and detect the intermediate, fill, freeze-dry, cover, test, and package to obtain carbo Sodium sulfonate freeze-dried powder injection finished product.
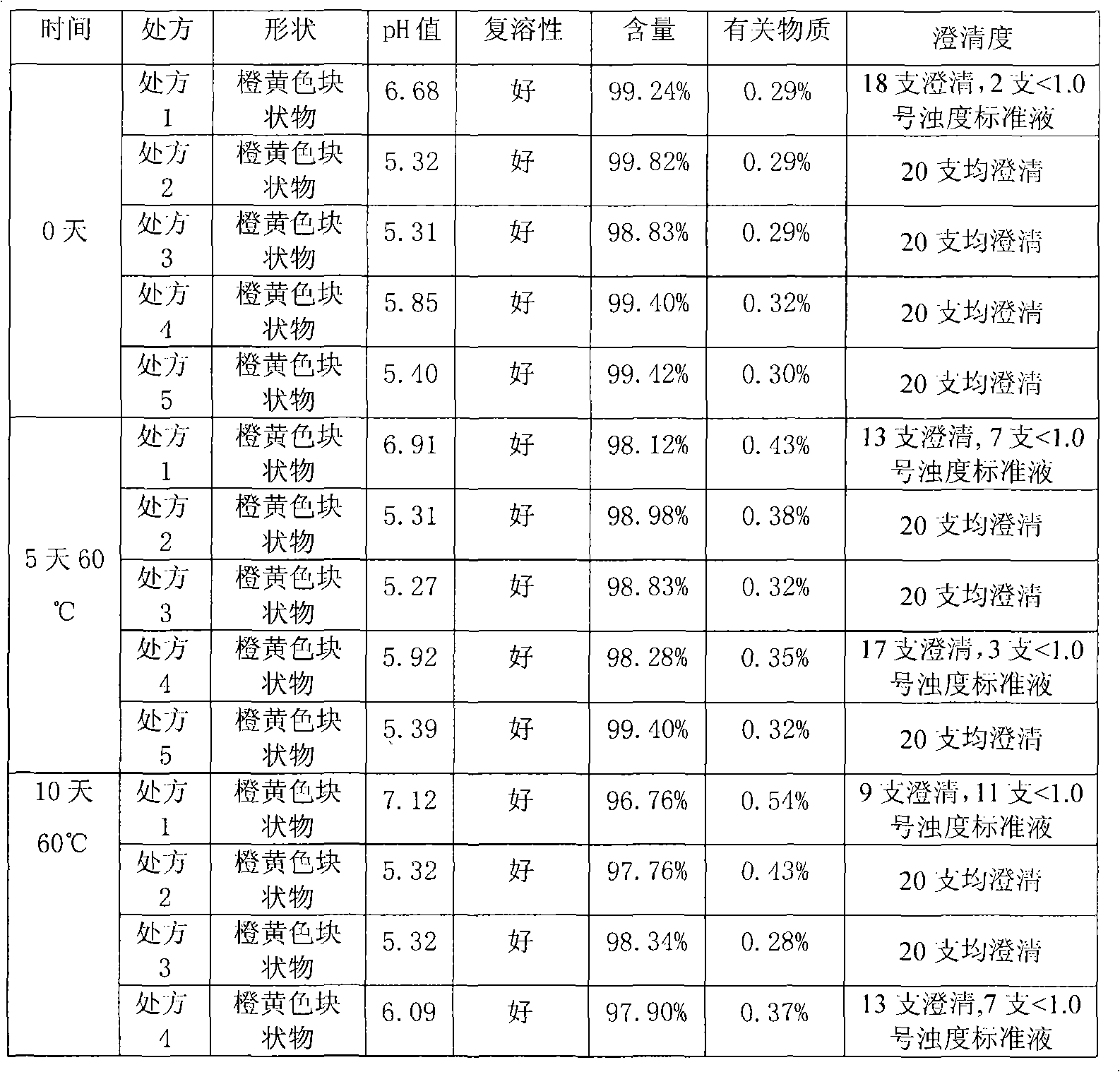
[0068] The method of the stability guidelines in the two appendices of the Chinese Pharmacopoeia 2005 edition was used to investigate the stability of the freeze-dried powder of sodium carbosulfon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com