Carbazochrome sodium sulfonate injection agent and preparation method thereof
A sodium carboxysulfonate injection and a technique for sodium carboxylate are applied in the field of medicine and can solve problems such as increased clinical adverse reactions, and achieve the effects of solving poor solubility and stability, good stability and few adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
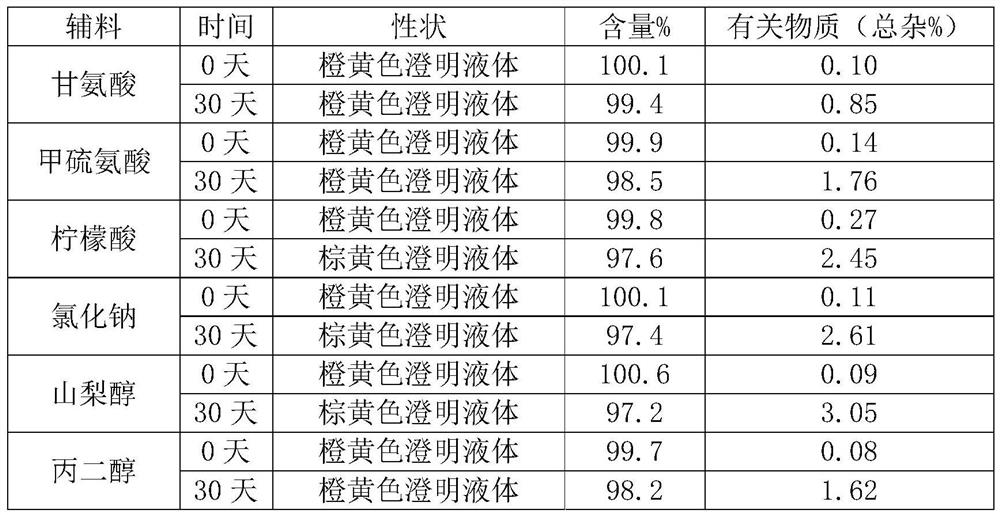
[0023] Prescription: Sodium carbosulfonate 100g, glycine 8g, add water for injection to 20L.
[0024] Preparation Process:
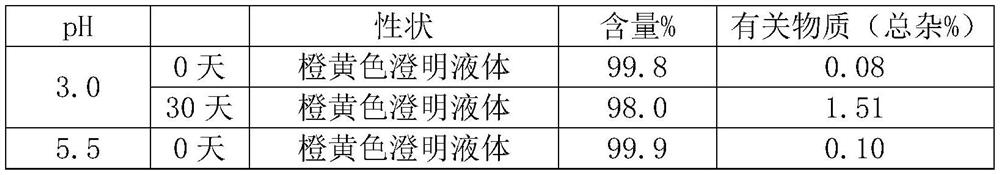
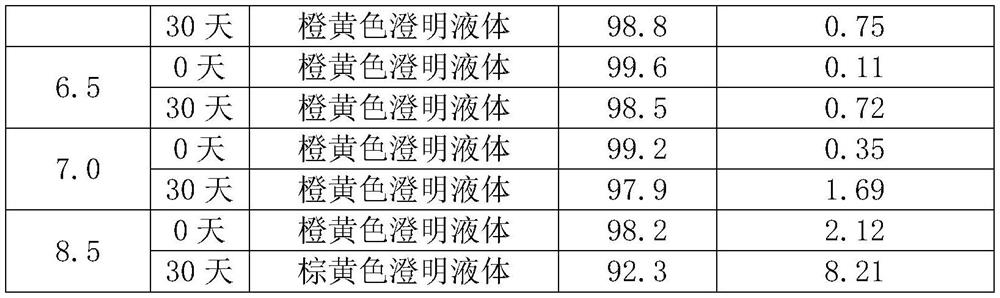
[0025] 1) Dissolve the prescribed amount of glycine in 90% water for injection (25°C), adjust the pH to 5.5 with hydrochloric acid, and stir for 5 minutes;
[0026] 2) After the step 1) is mixed evenly, add the raw material of sodium carbosulfonate, and stir for 15 minutes.
[0027] 3) Add the remaining water for injection to make up the volume, then turn on the circulating filtration system to perform one-stage 0.45um and two-stage 0.22um filtration, and the material of the filter element is polyethersulfone.
[0028] 4) Potting.
[0029] 5) Use non-terminal sterilization method for sterilization and filtration.
Embodiment 2
[0031] Prescription: Sodium carbosulfonate 100g, glycine 12g, add water for injection to 20L.
[0032] Preparation Process:
[0033] 1) Dissolve the prescribed amount of glycine in 90% water for injection (40°C), adjust the pH to 6.5 with hydrochloric acid, and stir for 3 minutes;
[0034] 2) After the step 1) is mixed evenly, add the raw material of sodium carbosulfonate, and stir for 5 minutes.
[0035] 3) Add the remaining water for injection to make up the volume, then turn on the circulating filtration system to perform one-stage 0.45um and two-stage 0.22um filtration, and the material of the filter element is polyethersulfone.
[0036] 4) Potting.
[0037] 5) Use non-terminal sterilization method for sterilization and filtration.
Embodiment 3
[0039] Prescription: Sodium carbosulfonate 100g, glycine 5g, add water for injection to 20L.
[0040] Preparation Process:
[0041] 1) Dissolve the prescribed amount of glycine in 90% water for injection (30°C), adjust the pH to 6.0 with hydrochloric acid, and stir for 4 minutes;
[0042] 2) After the step 1) is mixed evenly, add the raw material of sodium carbosulfonate, and stir for 10 minutes.
[0043] 3) Add the remaining water for injection to make up the volume, then turn on the circulating filtration system to perform one-stage 0.45um and two-stage 0.22um filtration, and the material of the filter element is polyethersulfone.
[0044] 4) Potting.
[0045] 5) Use non-terminal sterilization method for sterilization and filtration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com