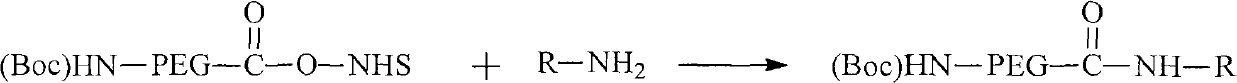Amphipathic compound using somatostatin analogue as target radical and pharmaceutics application thereof
A technology of amphiphilic compounds and somatostatin, which is applied in somatostatin, drug combinations, medical preparations of non-active ingredients, etc., can solve the problem of affecting drug efficacy, drug loss of targeting, and limiting the range of drug selection, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of octreotide-polyethylene glycol-stearic acid (OCT-PEG-R, R is an alkyl chain) conjugate:
[0059] 1. Preparation of octreotide-polyethylene glycol (OCT-PEG) conjugate
[0060] (Boc)HN-PEG-COO-NHS(PEG 5000 ) was dissolved in acetonitrile (MeCN), octreotide was dissolved in N,N-dimethylformamide (DMF), then the PEG solution was added dropwise to the octreotide solution, and triethylamine (TEA ), stirred at 4°C for 12 h. The reaction solution was poured into cold diethyl ether, the precipitate was filtered and the filter cake was washed with a small amount of diethyl ether, and the product was obtained by vacuum drying. The reaction solution can also be purified by HPLC, then diluted with water and freeze-dried to obtain (Boc)HN-PEG-OCT.
[0061] 2. Preparation of Octreotide-Polyethylene Glycol-Stearic Acid (OCT-PEG-R)
[0062] (Boc)HN-PEG-OCT was dissolved in trifluoroacetic acid (TFA) in acetonitrile (MeCN) solution, stirred at room temperature for 4 ho...
Embodiment 2
[0064] Octreotide succinate-polyethylene glycol-stearylamine ((S-OCT)-PEG-R, R is an alkyl chain) coupling preparation:
[0065] 1. Preparation of stearylamine-polyethylene glycol (R-PEG)
[0066] (Boc)HN-PEG-COO-NHS(PEG 5000 ) was dissolved in MeCN, stearylamine was dissolved in DMF, and then the PEG solution was added dropwise to the stearylamine solution, and TEA was added to the above mixed solution, and stirred at 4°C for 12h. The reaction solution was poured into cold diethyl ether, the precipitate was filtered and the filter cake was washed with a small amount of diethyl ether, and the product was obtained by vacuum drying. The reaction solution can also be purified by HPLC and then diluted with water and freeze-dried to obtain (Boc)HN-PEG-R.
[0067] 2. Preparation of octreotide succinate-polyethylene glycol-stearylamine ((S-OCT)-PEG-R)
[0068] (Boc)HN-PEG-R was dissolved in the MeCN solution of TFA, stirred at room temperature for 4h, the reaction solution was dil...
Embodiment 3
[0070] Octreotide-polyethylene glycol-stearic acid (OCT-PEG-R) modified N-deoxycholic acid-N, O-hydroxyethyl chitosan polymer micelles loaded with doxorubicin
[0071] 1. Preparation of N-deoxycholic acid-N, O-hydroxyethyl chitosan micelles loaded with doxorubicin (ADR)
[0072] Take the carrier N-deoxycholic acid-N, O-hydroxyethyl chitosan 17mg, accurately weighed, add 3mL of water, swell at 50°C for 1h, add the ADR solution neutralized by TEA drop by drop, stir at room temperature, and sonicate in an ice bath After 30 minutes, filter to obtain the drug-loaded micellar solution.
[0073] 2. Preparation of drug-loaded micelles modified by OCT-PEG-R
[0074] Take 1 mg of R-PEG-OCT, dissolve it in water, add it dropwise to the above micellar solution, stir at room temperature for 30 minutes, and sonicate in an ice bath for 10 minutes. The obtained micellar solution was dialyzed against water for 6 hours to obtain a drug-loaded solution with tumor targeting and long-circulation f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com