Preparation of glycollic acid from oxalaldehyde by intramolecular disproportionation method
A technology of glycolic acid and glyoxal, applied in carboxylate preparation, carboxylate preparation, organic chemistry, etc., can solve the problems of cumbersome process, high production cost, and low product purity, and achieve high reaction selectivity, The effect of less impurities and high purity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Add 40g of calcium hydroxide and 500ml of water into a 1000ml three-necked reaction flask (the mass percent concentration of the calcium hydroxide solution is 7.4%), take a water bath, stir mechanically, and drop 145g of glyoxal with a mass concentration of 40% from the dropping funnel Solution, the rate of addition is preferably to control the reaction temperature not to exceed 25°C, and after the dropwise addition is completed, keep the reaction at 20-25°C for 1 hour to obtain a suspended calcium glycolate precipitation mixture. While stirring, add 55g of concentrated sulfuric acid (0.54mol) to the suspended calcium glycolate precipitation mixture through the dropping funnel for acidification, filter out the calcium sulfate precipitate, and when the filtrate is vacuum concentrated to no water, a small amount of calcium sulfate dissolved in the filtrate precipitates out , separated by filtration, the filtrate was diluted with deionized water until the mass concentration ...
Embodiment 2
[0031] Add 40g of calcium hydroxide and 300ml of water (mass percentage concentration is 11.8%) in 1000ml three-necked reaction flask, water bath, mechanical stirring, dropwise add 145g of mass concentration to be 40% glyoxal solution from the dropping funnel, the rate of addition is It is advisable to control the reaction temperature not to exceed 25°C. After the dropwise addition is completed, keep the reaction at 20-25°C for 1 hour to obtain a suspended calcium glycolate precipitation mixture. The reaction mixture was filtered, and the filter cake was washed with deionized water to obtain a precipitate of calcium glycolate. The precipitate is acidified with 108g mass concentration of 50% sulfuric acid solution, the calcium sulfate precipitate is filtered out, and the filtrate is removed with a mass concentration of 10% barium acetate solution to remove trace SO 4 2- ions until no new BaSO 4 until precipitation occurs. Add 1g of powdered wood activated carbon for decolori...
Embodiment 3
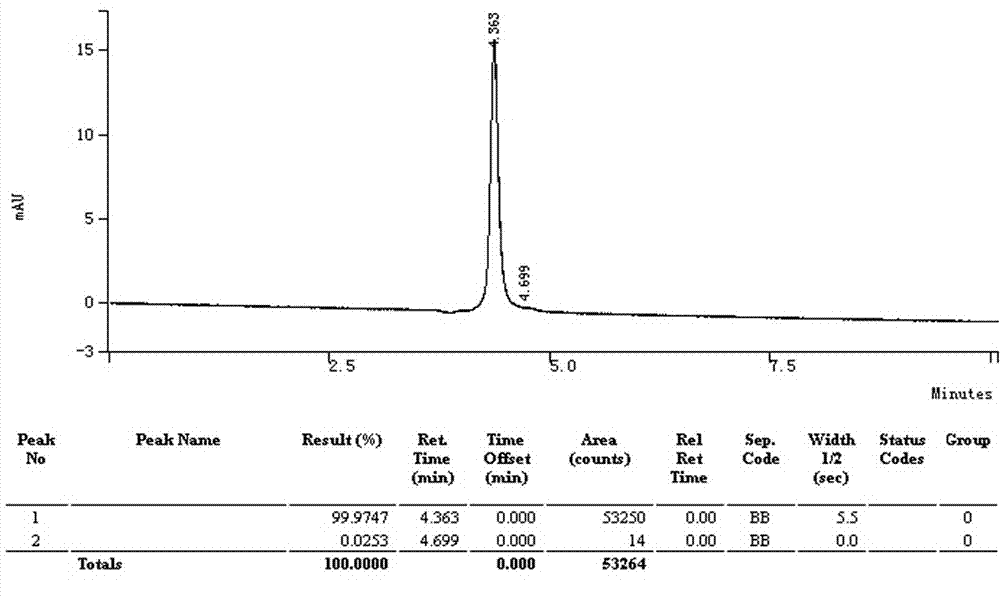
[0033] The glycolic acid solution obtained in Example 1 was concentrated by rotary evaporation until the mass concentration of glycolic acid was above 85%, the rotary evaporation temperature was 50-65°C, the vacuum degree was 0.080-0.095MPa, and cooled to room temperature, that is, crude glycolic acid crystals were precipitated. Filter, wash the crystals twice with a glycolic acid solution with a mass concentration of 60% as a detergent, and dry under reduced pressure in vacuum to obtain refined glycolic acid crystals. HPLC detection shows that the content of glycolic acid has a purity of more than 99%, and the yield of crystal glycolic acid is more than or equal to 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com