Production and application of high-stability recombination carboxypeptidase B
A carboxypeptidase and recombinant protein technology, which is applied in the production and application fields of recombinant carboxypeptidase B, and can solve the problem that proteins cannot spontaneously complete the folding process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1, the recombinant expression of carboxypeptidase B
[0069] 1. Recombinant expression of carboxypeptidase B (procarboxypeptidase B) with a leader sequence
[0070] Design the following primers:
[0071] Forward: 5'GCG CCA TGG CAT GCT TCC GAG GAG CAC TTT GATGGC-3' (SEQ ID NO: 3), containing Nco I restriction enzyme site;
[0072] Reverse: 5'-CGC AAG CTT TCA CTA ATA TAG ATG TTC TCG GAC ATAATT-3' (SEQ ID NO: 4), containing a Hind III restriction enzyme site and a stop codon.
[0073] Using the pancreatic cDNA library (purchased from Invitrogen) as a template, the aforementioned primers were used to perform PCR amplification to obtain the coding sequence of carboxypeptidase B with a leader sequence.
[0074] The sequence obtained above was digested with Nco I / HindIII and inserted into the corresponding site of the pET expression vector (purchased from Invitrogen), and the correctly inserted recombinant expression vector was identified by sequencing. The...
Embodiment 2
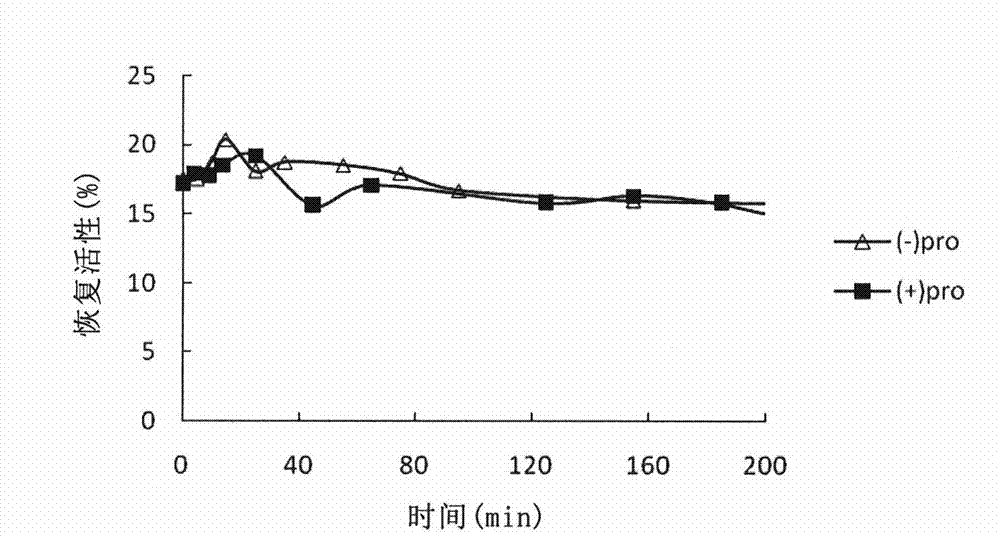
[0090] Example 2, the role of the leader sequence as an intermolecular chaperone on the folding of carboxypeptidase B
[0091] 1mg / ml purified recombinant carboxypeptidase B was denatured in 8M urea for 2h, and directly diluted 10-fold into refolding buffer (100mM Gly-NaOH, pH 9.5, GSH 1mM, 0.1mM ZnCl 2 ), the final protein concentration in the renaturation buffer is 0.1 mg / ml. Start timing from dilution and renaturation, and sample 0.1ml at regular intervals to measure activity. 100% enzyme activity is defined as the activity of the original enzyme solution (1 mg / ml) diluted 10 times directly at pH 9.5 with 0.1MGly-NaOH.
[0092] The activity assay uses hippuryl-L-arginine as the substrate, and the activity unit is defined as the percentage of substrate hydrolysis per minute at a substrate concentration of 0.001M. The activity buffer is: 0.025M Tris-HCl, pH7. 65 with 0.1M NaCl.
[0093] According to the molar ratio of CPB:pro=1:1, the leader sequence (pro) was added to the...
Embodiment 3
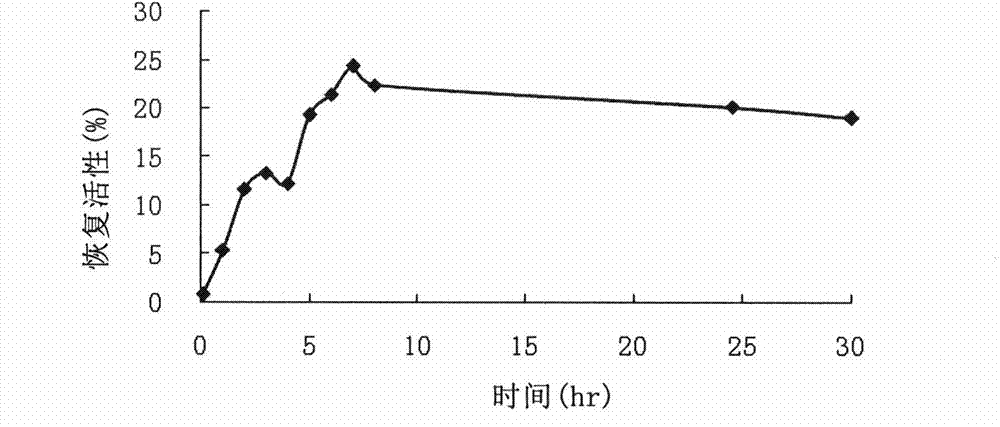
[0095] Example 3, the refolding effect of the leader sequence as an intramolecular chaperone of CPB on carboxypeptidase B
[0096] 0.5 mg / ml of the refolded and purified zymogen proCPB (recombinant carboxypeptidase B with a leader sequence, obtained after refolding without trypsin enzymatic activation and purified) was denatured in 8M urea for 24 hours, and directly Dilute 5 times into the refolding buffer, refold at room temperature, and the final protein concentration at this time is 0.1mg / ml. Start timing from dilution and renaturation, take 1ml at intervals, immediately add trypsin for enzymatic activation, wherein the mass ratio of procarboxypeptidase B: trypsin is 10:1, and measure CPB activity after incubation at 37°C for 40min. 100% enzyme activity is defined as the activity determined after the original enzyme solution (1 mg / ml) is diluted 5 times and activated by enzymatic hydrolysis in the same method.
[0097] The zymogen in the form of inclusion body (that is, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com