Recombinant human endostatin nanoparticle composition for injection and preparation method thereof
A technology of vascular endothelial and composition, applied in the field of recombinant human vascular endostatin nanoparticle composition for injection and its preparation, can solve the problems of complex production process, loss of drug activity, high quality requirements, etc., and achieve low cost, high protein The effect of little effect on activity and simple and feasible process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
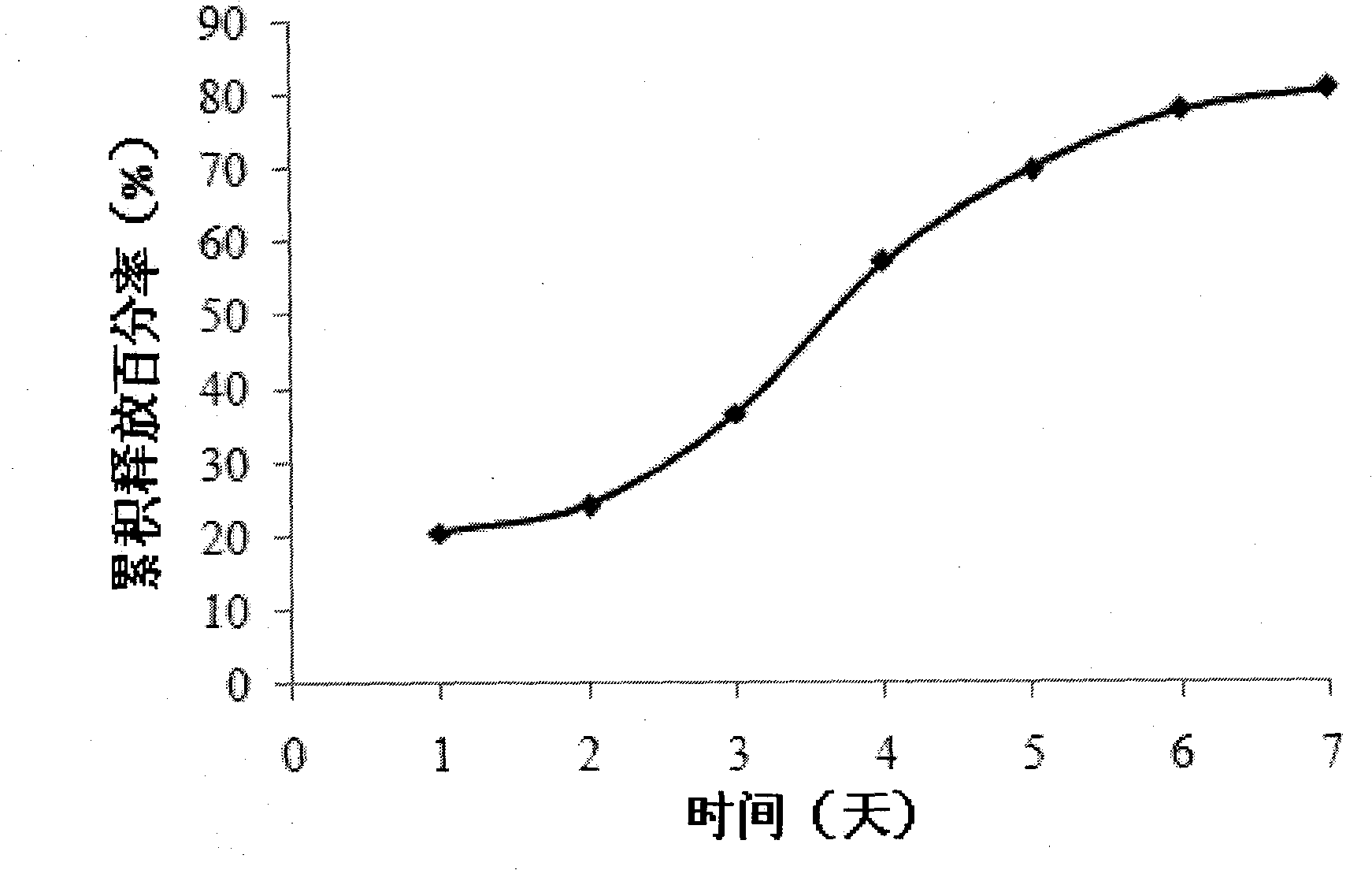
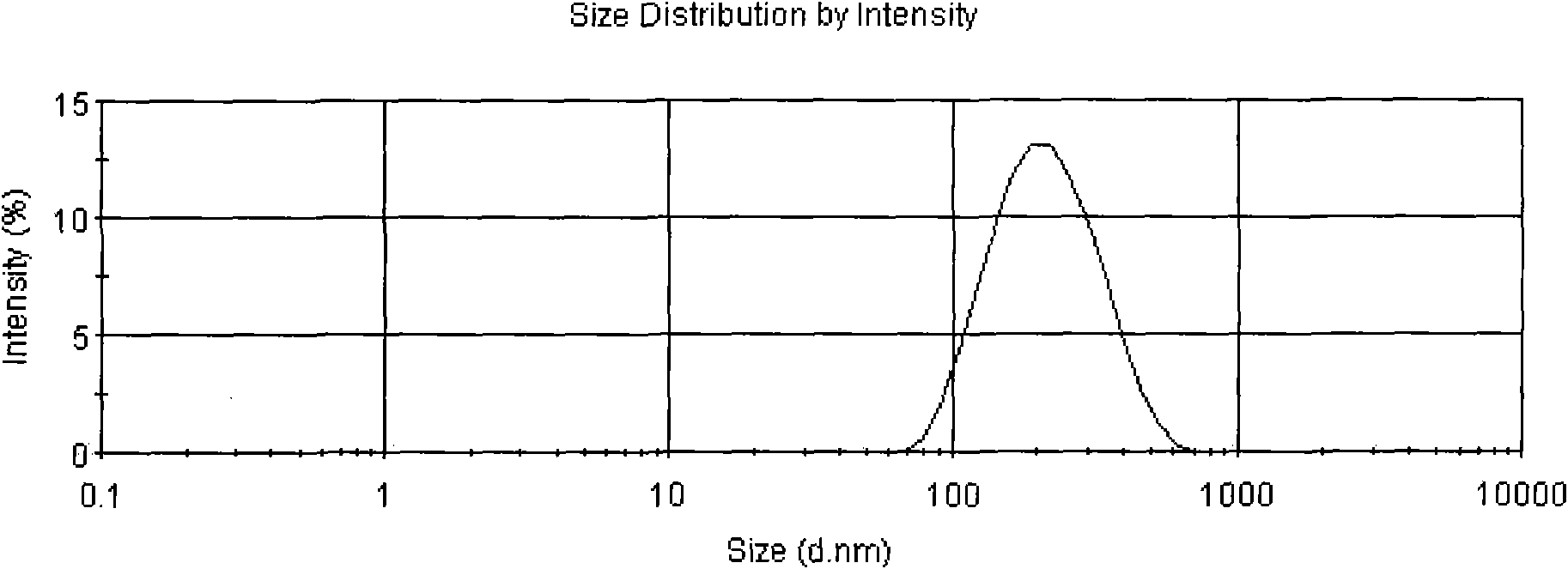
[0026] Weigh 100 mg of carboxy chitosan with a molecular weight of 100,000 Daltons and dissolve it in 50 ml of Span-80 solution. Prepare 4ml of 5mg / ml sodium sulfate solution with distilled water. Add 30 mg of Endostar acetate buffer solution (pH5.5) into the chitosan solution, slowly add sodium sulfate solution dropwise under magnetic stirring, react for 30 minutes, ultrasonicate for 5 minutes at 100 W power, and centrifuge at 10,000 rpm for 30 minutes. Minutes to separate the nanoparticles. The obtained nanoparticles were redispersed in 50 ml of water, 1 g of fructose was added, and freeze-dried to obtain recombinant human endostatin nanoparticles. The drug loading of the obtained nanoparticles was 17.1%.
Embodiment 2
[0028]Weigh 500 mg of carboxymethyl chitosan with a molecular weight of 200,000 Daltons and dissolve it in 50 ml of 1% acetic acid solution. Prepare 20ml of 5mg / ml sodium alginate solution with distilled water. Add 200 mg Endostar acetate buffer solution (pH5.5) into the chitosan solution, slowly add sodium alginate solution dropwise under magnetic stirring, react for 60 minutes, and centrifuge the above solution at 4 degrees Celsius and 10,000 rpm For 30 minutes, the nanoparticles were isolated. The obtained nanoparticles were redispersed in 50 ml of water, 1 g of sucrose was added, and freeze-dried to obtain recombinant human endostatin nanoparticles. The drug-loaded weight of the obtained nanoparticles is 20.7%.
Embodiment 3
[0030] Weighing 250 mg of chitosan with a molecular weight of 150,000 Daltons was dissolved in 50 ml of 1% acetic acid solution to obtain a 5 mg / ml chitosan solution. Prepare 20ml of 2.5mg / ml sodium tripolyphosphate solution with distilled water. Add 100mg of Endostar phosphate buffer (pH5.5) and 10ml of polyethylene glycol into the chitosan solution, slowly add sodium tripolyphosphate solution dropwise under magnetic stirring, react for 10 minutes, and ultrasonicate the probe for 2 minutes at 100W power, and put The above solution was centrifuged at 4°C and 10,000 rpm for 30 minutes to separate nanoparticles. The obtained nanoparticles were redispersed in 50 ml of water, 1.5 g of trehalose was added, and freeze-dried to obtain recombinant human endostatin nanoparticles. The drug loading of the obtained nanoparticles was 22.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com