Irinotecan nano circulating liposome and preparation method thereof
A nano-liposome and irinotecan technology, applied in the field of biomedicine, can solve the problems of low encapsulation rate, instability, uneven particle size distribution of liposomes, etc., and achieves easy industrialization, is conducive to absorption, and solves The effect of uneven particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of irinotecan long-circulation nanoliposomes.
[0040] Select PEG-DSPE as an additional agent for preparing long-circulating liposomes, and phospholipids, cholesterol, poloxamer 188, V E Dissolved in an ethanol solution, injected into the ammonium sulfate solution in which PEG-DSPE was dissolved under the condition of stirring in a water bath at 55°C, the concentration of phospholipids was 100 mg / ml, and the mass ratio of phospholipids, cholesterol, poloxamer 188, and PEG-DSPE was 3: 1:0.6:0.3, pass N 2 Under the condition of incubating and stirring for 0.5-1h, the obtained long-circulation blank liposomes were dialyzed overnight in normal saline, the pH of the external phase was adjusted to be 7-7.5, and the irinotecan solution was added under the condition of 55°C water bath (irinotecan / phospholipid mass ratio 1 :10) Incubate for 5-15min to obtain irinotecan long-circulation nanoliposomes.
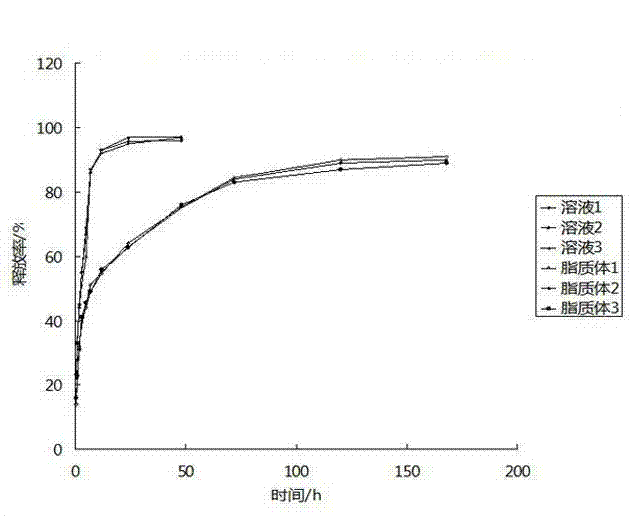
[0041] The irinotecan long-circulation nanoliposomes...
Embodiment 2
[0045] Embodiment 2, the preparation of irinotecan long circulation nano liposome
[0046] Select PEG2000 as an additional agent for preparing long-circulating liposomes, and phospholipids, cholesterol, poloxamer 188, V E Dissolved in ethanol solution, injected into the ammonium sulfate solution in which PEG2000 was dissolved under the condition of stirring in a water bath at 55°C, the concentration of phospholipids was 100 mg / ml, and the mass ratio of phospholipids, cholesterol, poloxamer 188, and PEG2000 was 10:5:2: 1, pass N 2 Under the condition of incubating and stirring for 0.5-1h, the obtained long-circulation blank liposomes were dialyzed overnight in normal saline, the pH of the external phase was adjusted to 7.5, and irinotecan solution was added under the condition of 55°C water bath (irinotecan / phospholipid mass ratio 1:10 ) was incubated for 5-15 minutes to obtain irinotecan long-circulation nanoliposomes.
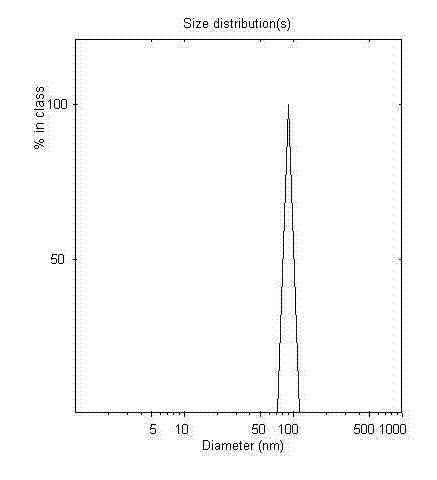
[0047] The particle size of irinotecan long-circulatio...
Embodiment 3
[0048] Embodiment 3, the preparation of irinotecan long circulation nano liposome injection
[0049] The obtained irinotecan long-circulation nanoliposomes prepared in Examples 1 and 2 are filtered and sterilized by a microporous membrane, filled with nitrogen and filled in an ampoule vial to obtain the injection of irinotecan long-circulation nanoliposomes liquid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com