Compositions and methods for preventing transepithelial transmission of hiv
A composition, epithelial technology, applied in the field of application of ICAM-1 agonist/antagonist to prevent HIV from spreading through mucosal surfaces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Isolation of cell-free HIV and HIV-infected peripheral blood lymphocytes and monocytes
[0082] HIV-1 Ba-L (Advanced Biotechnologies Inc., Columbia, MD), a macrophage-tropic, NSI, CCR5-applied variant of HIV, is commercially available as 1.0 ml aliquots (1 x 10 6 50% tissue culture infectious dose (TCID 50 ) / ml) and stored in liquid nitrogen. Virus stocks were freshly thawed before application to cultures.
[0083] Human PBMCs were isolated by centrifugation of leukapheresis-processed blood on Ficoll Hypaque (Pharmacia, Piscataway, NJ). Total PBMCs were treated with RPMI-1640 supplemented with 100U / ml penicillin / 100mcg streptomycin, 10ng / ml gentamycin, and 2mM L-glutamine (medium and all supplements were from Life Technologies, Grand Island, NY) diluted to 1×10 7 / ml, then plated at 75 or 150cm 2 Tissue culture flasks (Corning Scientific Products, Inc., Cambridge, MA). During the first 12 hours of culture, cells were resuspended in 20% heat-inactivated FCS (56°...
Embodiment 2
[0087] Transwell culture of human cervical epithelial cells
[0088] The spontaneously transformed human cervical epithelial cell line ME-180 (ATCC, Rockville, MD) was cultured in a 75 cm 2 Cells were routinely passaged every 3 days by treating them with 0.05% trypsin-EDTA (Life Technologies) in cRPMI-10% FCS in flasks. ME-180 cells in 2 × 10 5 / 0.1 ml cRPMI-10% FCS or -10% HS / 5.0 μm was plated on a 12 mm diameter transwell insert (Corning Scientific Products, Inc.). Maintain ME-180 cells in trans wells at 37 °C, 5% CO 2 Down. Change the medium every 2-3 days. After 7 days, cells formed a polarized complete monolayer on the transwell insert, which was confirmed by monitoring the impedance across the epithelium using an ohmmeter (Millipore) and staining with a fluorochrome-labeled anti-ZO1 antibody, where ZO1 is a protein found in tight junctions of epithelial cells (20) (1:50 dilution, Zymed, San Francisco, CA). The ME-180 monolayer on the insert is ready to use for ex...
Embodiment 3
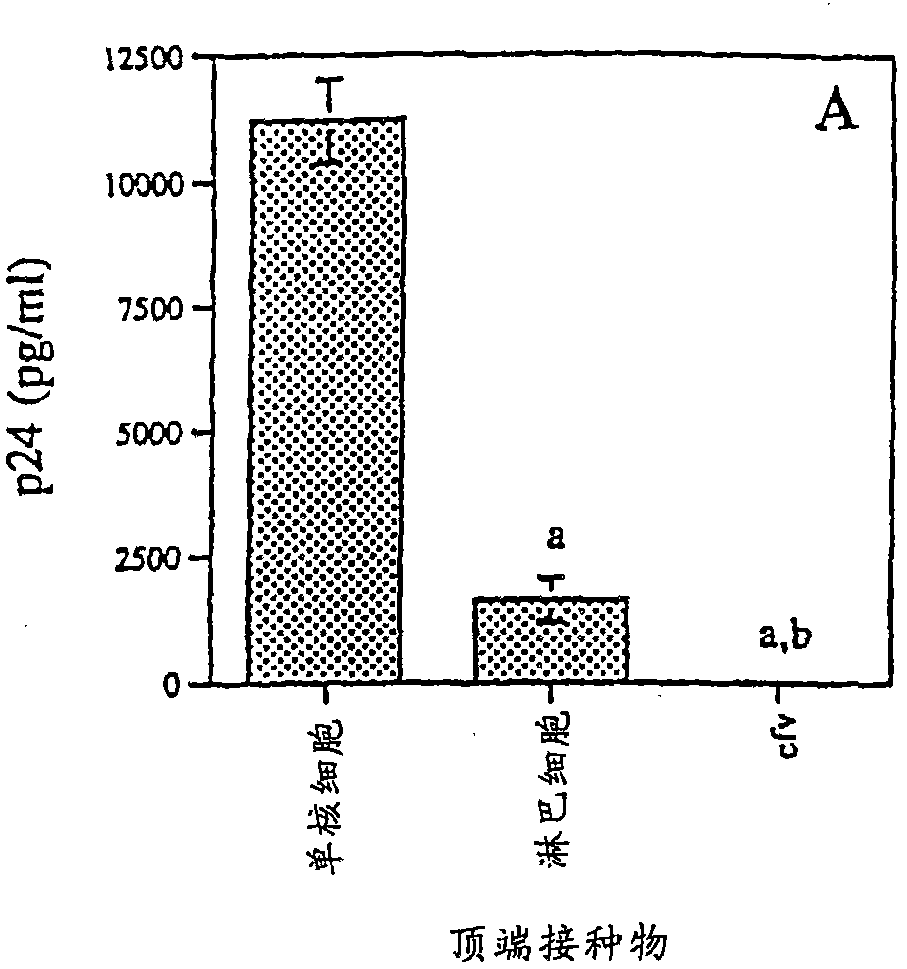
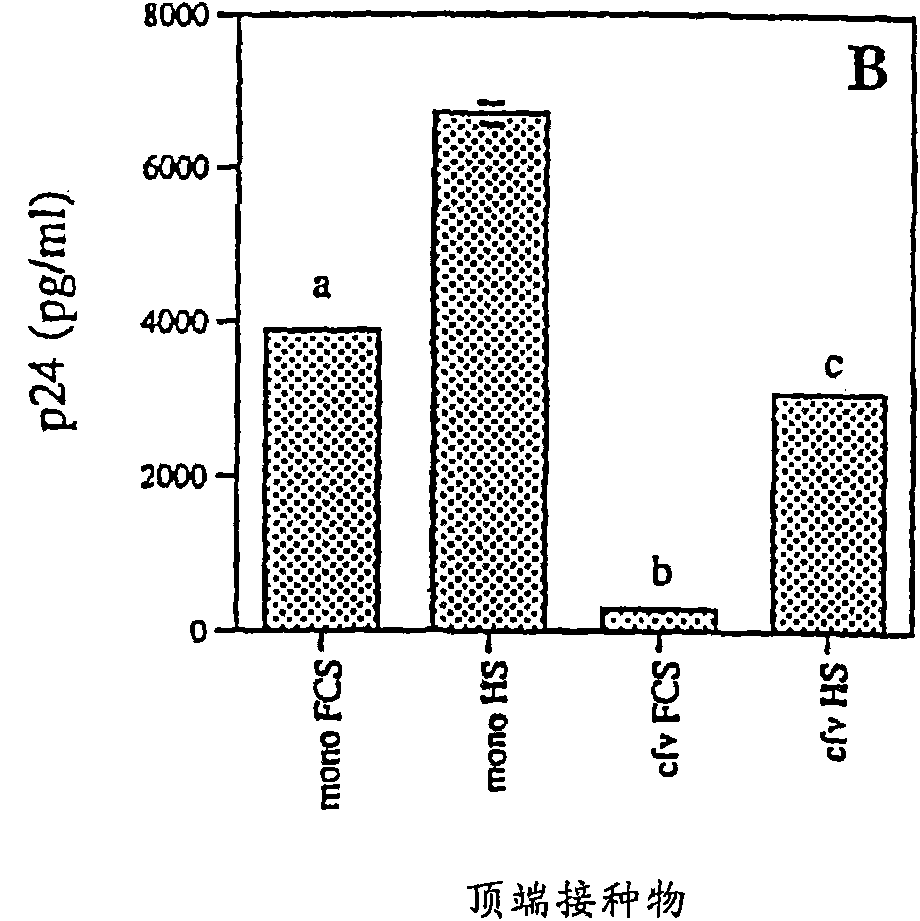
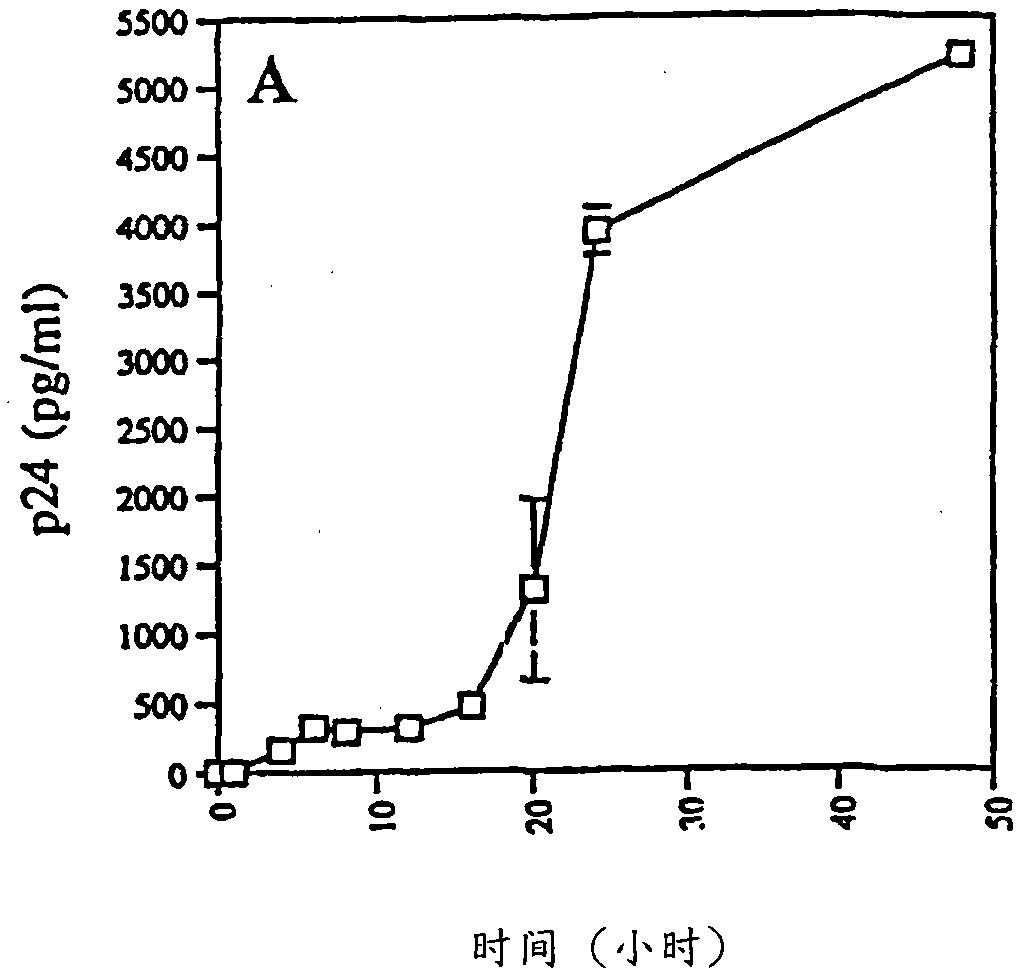
[0090] Transepithelial migration and HIV-1 transmission
[0091] Transepithelial migration assays were performed as described by Bomsel with several modifications. In short, the 1×10 6 HIV-1 Ba-L Infected monocytes, 1×10 6 HIV-1 Ba-L Infected PBLs, or monocytes or cell-free HIV-1 in PBL culture supernatants were added to the apical side of the epithelial cell monolayer. A time-course analysis was performed, with media collected on the apical and basal sides between 1-48 hours after addition of infected cells to the inserts. Spread across the monolayer approaches a maximum at 24 hours, the time point chosen for all subsequent experiments. The viability of monocytes and PBLs was assessed by trypan blue (Sigma) exclusion before adding cells to transwells and results were always >90% after 24 hours of propagation. The amount of HIV-1 p24 antigen in the apical and basolateral supernatants was quantified by HIV-1 p24 antigen ELISA assay (Dupont NEN) (sensitivity 12.5 pg / ml-350...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com