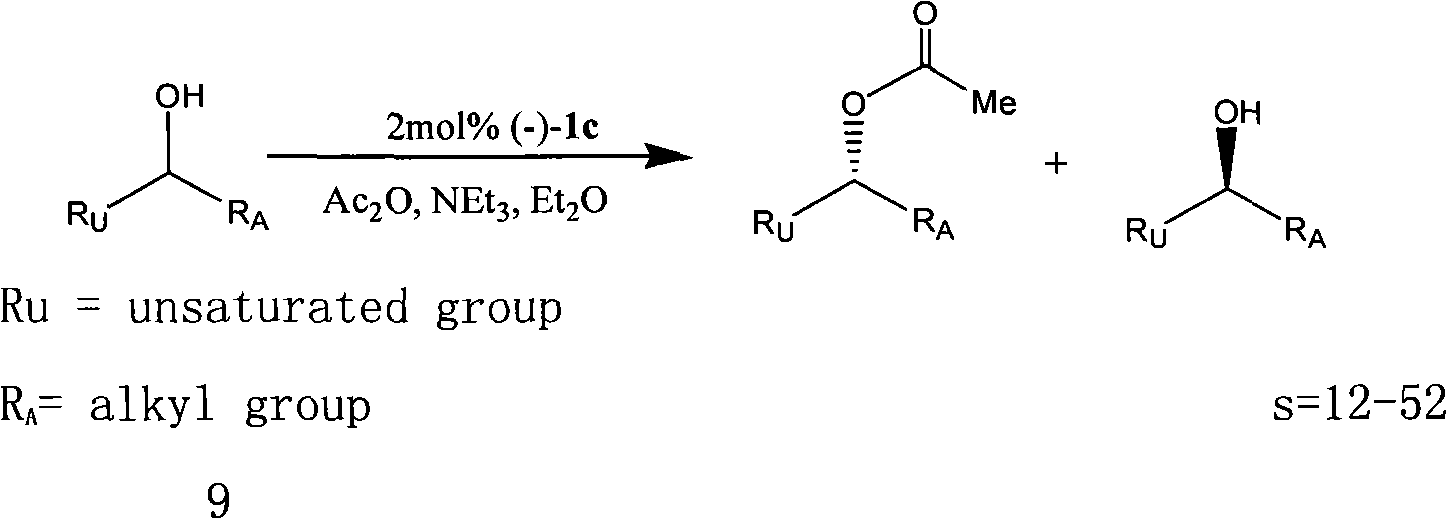4-(N,N-dimethyl) aminopyridine derivate and synthesis method thereof
A technology of dimethylaminopyridine and aminopyridine is applied in the field of 4-aminopyridine derivatives and synthesis thereof, and can solve the problems of high synthesis cost and remote practical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Add 0.126g (0.60mmol) of 3-bromo-4-(N,N-dimethyl)aminopyridine into a 5mL polytetrafluoroethylene autoclave, then add 0.030mmol triphenylphosphorous palladium catalyst, 3.0 1 mmol olefinic compound and 6.0 mmol triethylamine, put the autoclave into an oven and heat to 120° C., and react for 24 hours. After stopping the reaction, cool to room temperature, add 10mL of dichloromethane and 10mL of water, separate the organic layer, extract with dichloromethane (10mL x 3), dry the organic layer with anhydrous sodium sulfate, filter the organic phase, and remove it under reduced pressure. solvent, and the residue was purified by thin layer chromatography to obtain the product. For the alkenes used and the reaction results, please refer to Table 1
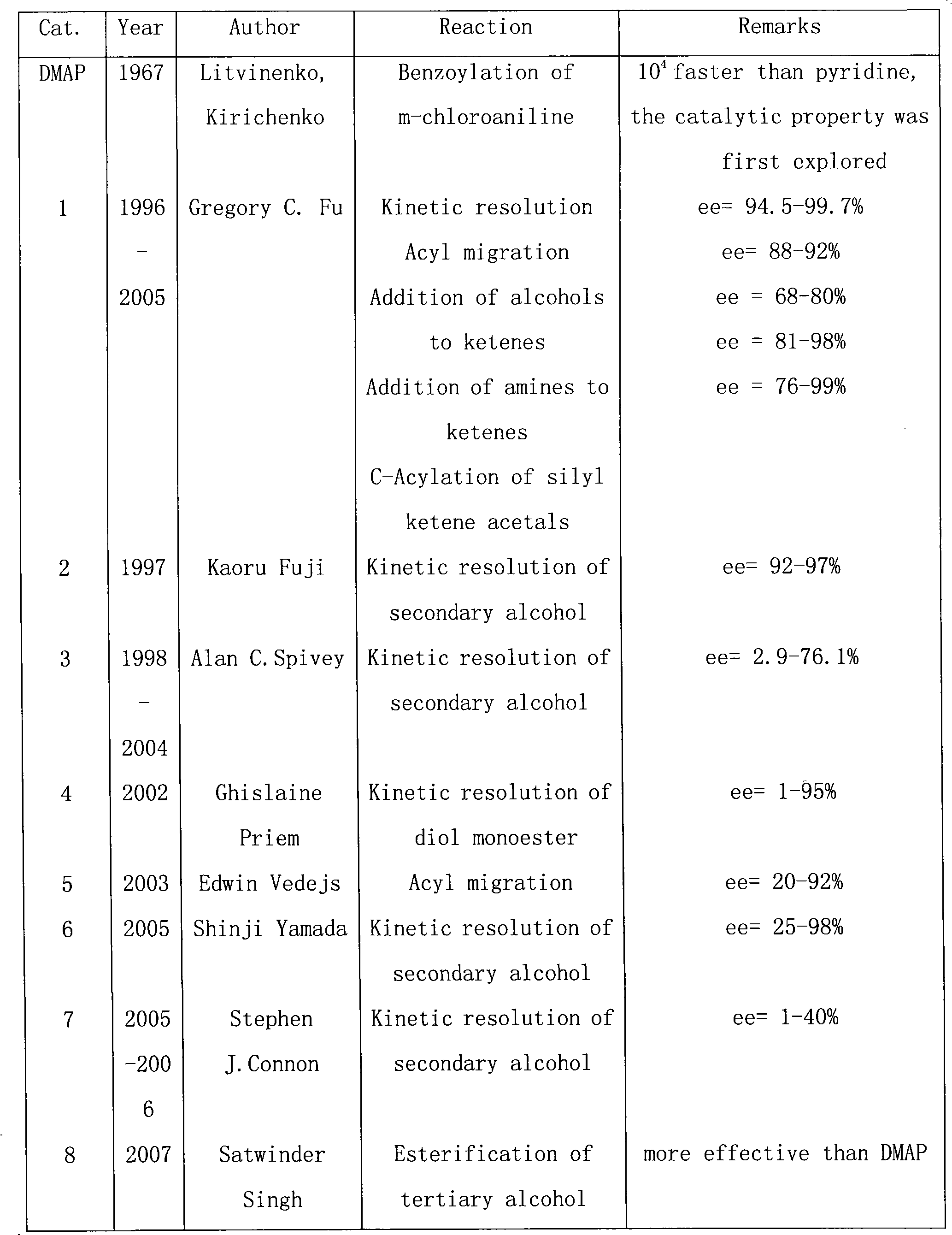
[0051] Table 1 Alkenes and their reaction results
[0052]
[0053] Characterization parameters of each product:
[0054] 1. The structural formula of product 1:
[0055] yellow liquid.
[0056] 1 H-NMR (CDCl ...
Embodiment 2
[0085] Example 2: Synthesis of (S)-3-(4-N,N-dimethylaminopyridine-3-)-N-(1-phenylethyl)-acrylamide
[0086] Add 0.100 g (0.50 mmol) of 3-bromo-4-(N,N-dimethyl)aminopyridine into a 5 mL polytetrafluoroethylene autoclave, then add 0.025 mmol Pd catalyst, 0.60 mmol (S)-N-( 1-phenylethyl)-acrylamide and 5.00 mmol of acid-binding agent triethylamine TEA, put the autoclave into an oven and heat to a certain temperature, and react for 24 hours. After stopping the reaction, cool to room temperature, add 10mL dichloromethane and 10mL water, separate the organic layer, then extract with dichloromethane (10mL x3), dry the organic layer with anhydrous sodium sulfate, filter the organic phase, and The solvent was removed, and the residue was purified by thin layer chromatography (dichloromethane:ethanol=9.8:0.2) to obtain the product (S)-3-(4-N,N-dimethylaminopyridine-3-)-N-(1 -phenylethyl)-acrylamide, productive rate 43%, its structural formula is:
[0087]
[0088] yellow solid.
...
Embodiment 3
[0091] Example 3: Synthesis of (S)-1-[3-(4-dimethylaminopyridine-3-)-acryloyl]-pyrrolidine-2-carboxylic acid ethyl ester 8:
[0092] Add 0.100 g (0.50 mmol) of 3-bromo-4-(N,N-dimethyl)aminopyridine into a 5 mL polytetrafluoroethylene autoclave, then add 0.025 mmol of Pd catalyst, 0.60 mmol of (S)-1-propylene Acylpyrrolidine-2-carboxylic acid ethyl ester and 5.00mmol acid-binding agent TEA, put the autoclave into an oven and heat to a certain temperature, and react for 24 hours. After stopping the reaction, cool to room temperature, add 10mL of dichloromethane and 10mL of water, separate the organic layer, then extract with dichloromethane (10mL x 3), dry the organic layer with anhydrous sodium sulfate, filter the organic phase, and depressurize The solvent was removed, and the residue was purified by thin layer chromatography (dichloromethane:ethanol=9.8:0.2) to obtain product 8, namely (S)-1-[3-(4-dimethylaminopyridine-3-)- Acryloyl]-pyrrolidine-2-carboxylic acid ethyl ester...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com