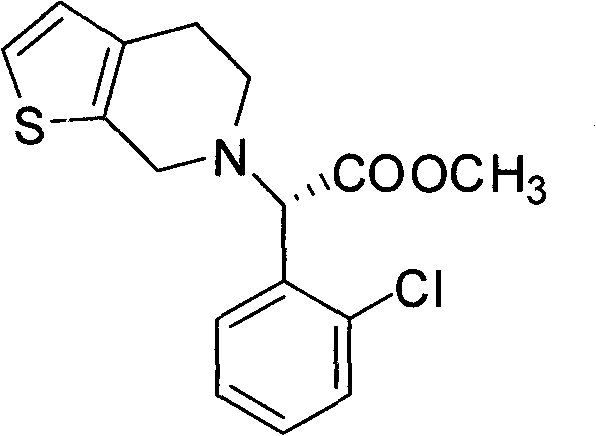
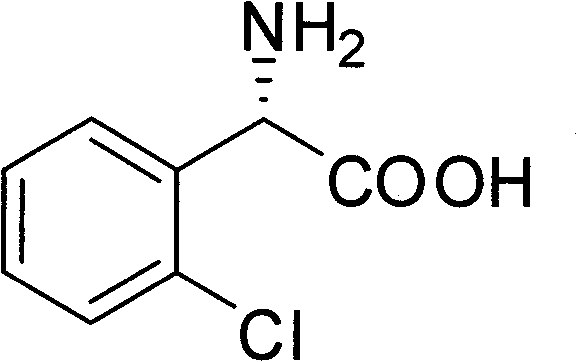
Chemical-enzyme method for preparing (S)-2-chlorophenyl glycine methyl ester clopidogrel chiral intermediate
A technology for o-chlorophenylglycine methyl ester and o-chlorophenylglycine, which is applied in the field of chemical-enzymatic methods for preparing a chiral intermediate of clopidogrel-o-chlorophenylglycine methyl ester, and can solve the problem of low optical purity and influence on end products. problems such as the optical purity of clopidogrel, achieving high optical activity, eliminating the recrystallization step, and simplifying the preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
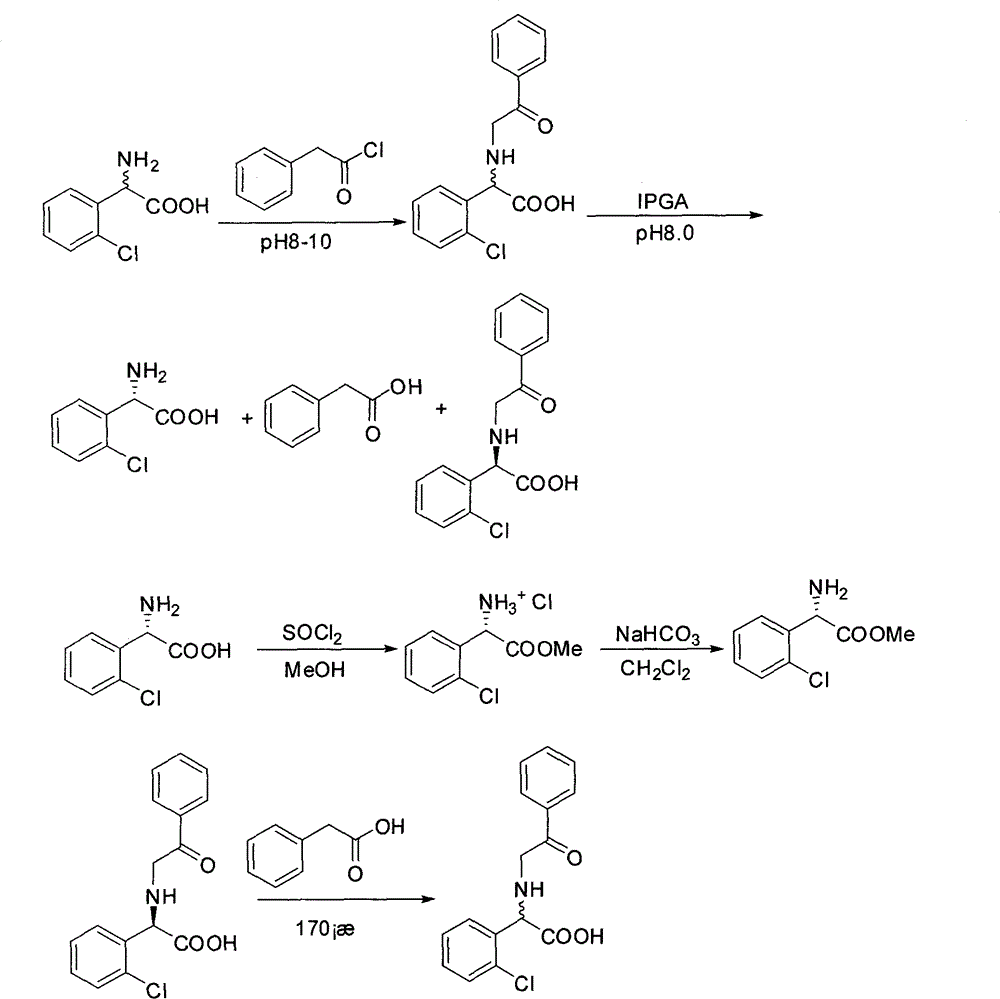
[0019] Embodiment 1: Preparation of (R, S)-N-phenylacetyl-o-chlorophenylglycine
[0020] Add 74.2g (0.4mol) (R, S)-o-chlorophenylglycine and 48g NaOH (1.2mol) into 700ml water, stir to dissolve. 64ml (0.48mol) of phenylacetyl chloride (or phenylacetic acid, or methyl phenylacetate, or phenylacetyl bromide) was added dropwise under ice-bath conditions. After the dropwise addition was completed, the reaction was carried out overnight at room temperature. Adjust the pH to 1-2 with hydrochloric acid, and precipitate (R, S)-N-phenylacetyl-o-chlorophenylglycine as a solid under stirring. Suction filtration and drying gave 115.6 g of (R, S)-N-phenylacetyl-o-chlorophenylglycine with a yield of 95%.
Embodiment 2
[0021] Example 2: (R, S)-N-phenylacetyl-o-chlorophenylglycine enzyme catalyzed hydrolysis
[0022] Add 91.2g (0.3mol) of (R,S)-N-phenylacetyl-o-chlorophenylglycine to 600ml of water, and adjust the pH to 8.0 with ammonia water. Add 18.3 g of immobilized penicillin acylase, and stir the reaction at 30° C. for 12 h. Remove immobilized penicillin acylase by suction filtration. The filtrate was adjusted to pH 1-2 with concentrated hydrochloric acid, filtered with suction, the solid was washed with hot water, and dried to obtain 44.4 g of (R)-N-phenylacetyl-o-chlorophenylglycine with a yield of 97%. The filtrate was concentrated under reduced pressure at 60°C, and the isoelectric point was adjusted to precipitate a solid. The solid was washed with absolute ethanol and dried to obtain 12.6 g of (S)-o-chlorophenylglycine with a yield of 90% and ee of 100%.
Embodiment 3
[0023] Embodiment 3: Preparation of (S)-o-chlorophenylglycine methyl ester hydrochloride
[0024] Add 18.6g (0.1mol) (S)-o-chlorophenylglycine to 200ml of anhydrous methanol, and add SOCl dropwise in an ice bath (0-5°C) 2 14.5ml. After the dropwise addition was completed, the reaction was carried out at room temperature for 5h. Evaporate to dryness under reduced pressure at 60°C, and dry in vacuo to obtain 23.1 g of (S)-o-chlorophenylglycine methyl ester hydrochloride, with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com