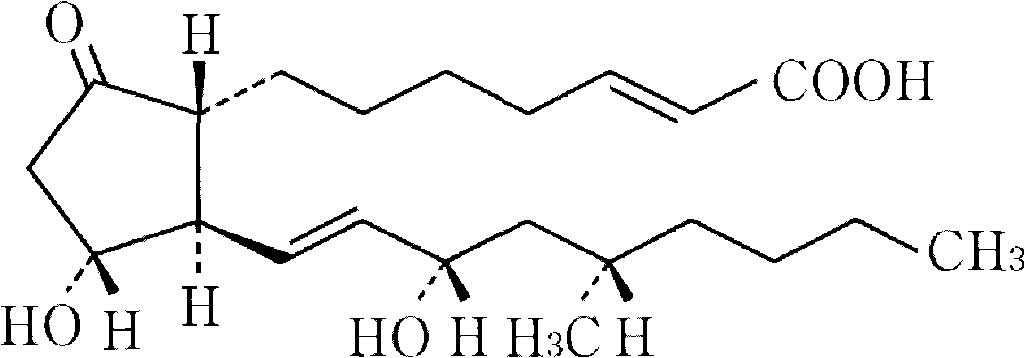
Drug composite containing limaprost and preparation method thereof
A technology for limaprost and a composition is applied in the field of oral limaprost tablets and the preparation thereof, which can solve the problem of no patent application for limaprost, and achieve improved bioavailability, uniform content and fast onset of action Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
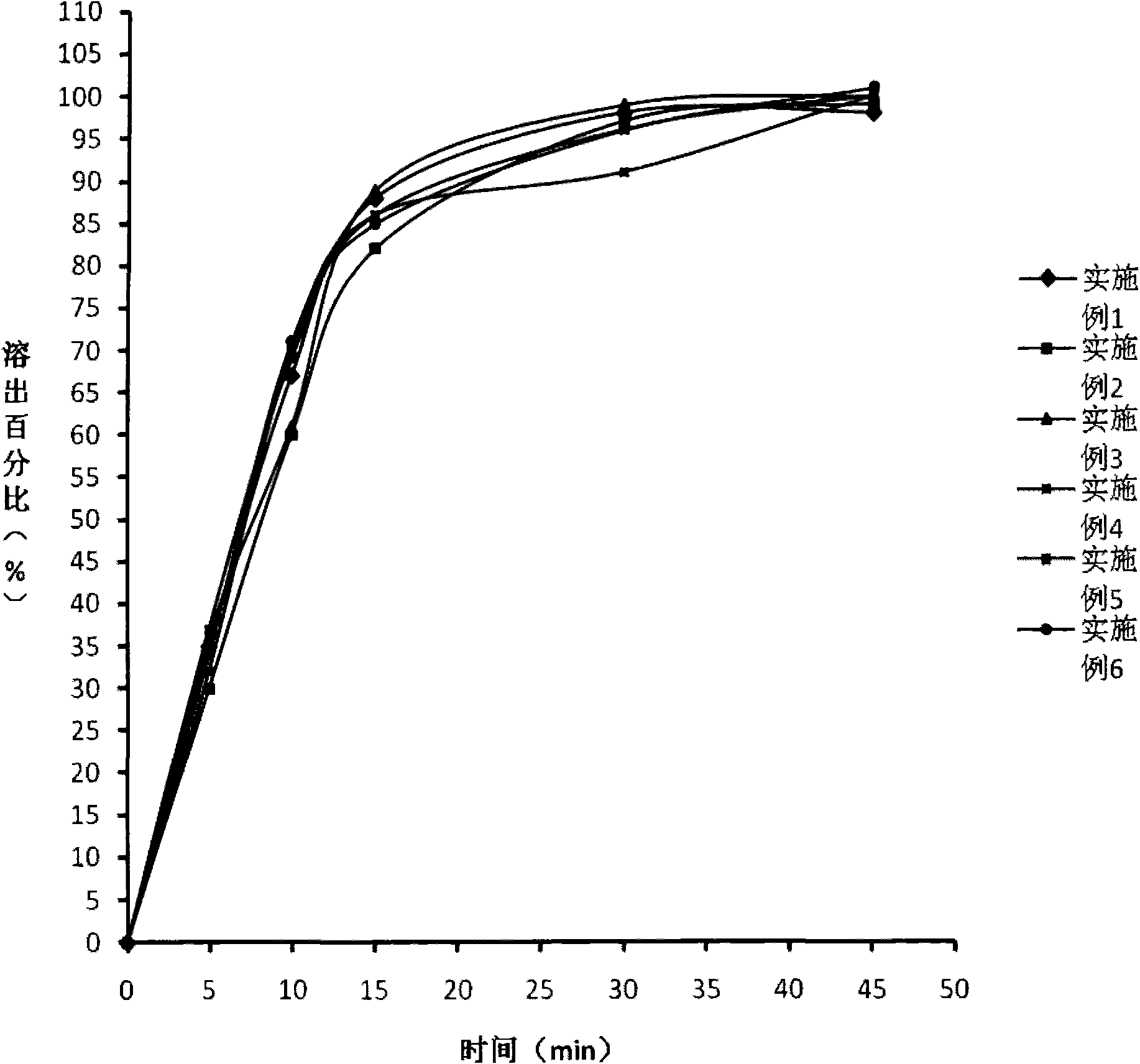
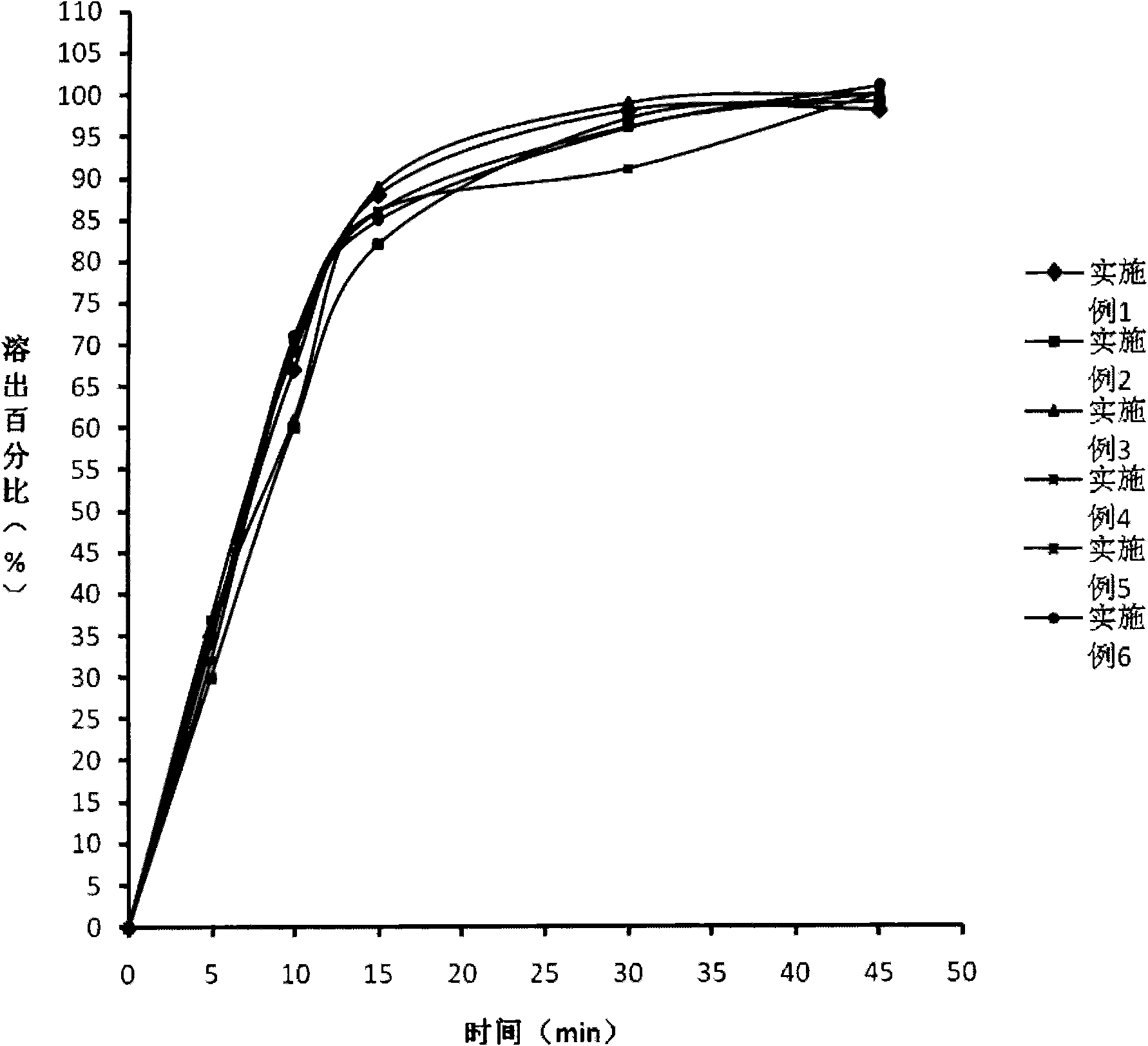
Embodiment 1
[0041] Pharmaceutical composition of the present invention--the prescription composition of limatoprost tablet:
[0042] Limaprost Hydroxypropyl-α-CD Inclusion Complex 0.17g
[0043] Mannitol 0.72g
[0044] Sorbitol 1.78g
[0046] Povidone 4.50g
[0047] Lactose 70.23g
[0049]
[0050] Made into tablets 85mg / tablet, made into 1000 pieces
[0051] Preparation:
[0052] a. Weigh 0.72g mannitol, 1.78g sorbitol and 0.17g limaprost hydroxypropyl-α-CD inclusion compound, dissolve with appropriate amount of water, freeze-dry, and pre-freeze at -40°C, 200Pa, - Vacuum at 20°C, dry at 100Pa and 20°C to obtain lyophilized mixture I;
[0053] b. Pulverize the freeze-dried mixture I, pass through a 100-mesh sieve, add 7.00g starch slurry, 4.50g povidone, and 70.23g lactose according to the prescription ratio, mix for more than 5 minutes, then add 0.30g of talcum powder, and mix fo...
Embodiment 2
[0065] The prescription composition of Limaprost tablet:
[0066] Limaprost Hydroxypropyl-γ-CD Inclusion Complex 0.0125g
[0067] Mannitol 0.70g
[0068] Trehalose 0.70g
[0069] Pectin 4.50g
[0070] Sodium carboxymethyl starch 8.00g
[0071] Maltose 70.08g
[0072] Magnesium Stearate 1.00g
[0073]
[0074] Made into tablets 85mg / tablet, made into 1000 pieces
[0075] Preparation:
[0076] a. Weigh 0.70g of mannitol, 0.70g of trehalose and 0.0125g of limatoprost hydroxypropyl-γ-CD inclusion compound, dissolve with an appropriate amount of water, freeze-dry, and pre-freeze at -30°C, 300Pa , Vacuum at -20°C, and dry at 200Pa at 20°C to obtain freeze-dried mixture I;
[0077] B, freeze-dried mixture I is pulverized, crosses 100 mesh sieves, adds 4.50g pectin, 8.00g carboxymethyl starch sodium, 70.08g maltose according to prescription ratio, mixes more than 5 minutes, then adds the magnesium stearate of 0.50g, Mix for more ...
Embodiment 3
[0086] The prescription composition of Limaprost tablet:
[0087] Limaprost-α-CD inclusion complex 0.25g
[0088] Mannitol 0.75g
[0089] Sucrose 0.25g
[0090] Hypromellose 10.00g
[0091] Low-substituted hydroxypropyl cellulose 10.00g
[0092] Sorbitol 63.25g
[0093] Micronized silica gel 0.50g
[0094]
[0095] Made into tablets 85mg / tablet, made into 1000 pieces
[0096] Preparation:
[0097] a. Weigh 0.75g of mannitol, 0.25g of sucrose and 0.25g of limatoprost-α-CD inclusion compound, dissolve with an appropriate amount of water, freeze-dry, and pre-freeze at -20°C, 65Pa, -30°C Under vacuum, 90Pa, 30°C drying to obtain freeze-dried mixture I;
[0098] b. Pulverize the freeze-dried mixture I, pass through a 100-mesh sieve, add 10.00g hydroxypropylmethylcellulose, 10.00g low-substituted hydroxypropylcellulose, and 63.25g sorbitol according to the prescription ratio, mix for more than 5 minutes, and then add 0.25g Micropowder ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com