Alprostadil submicron emulsion for injection and preparing method thereof
A technology of dierythia microemulsion and alprostadil, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve the problems of large fluctuations in the preparation process, large differences in product quality, and expiration dates. To achieve the effect of ensuring clinical equivalence and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
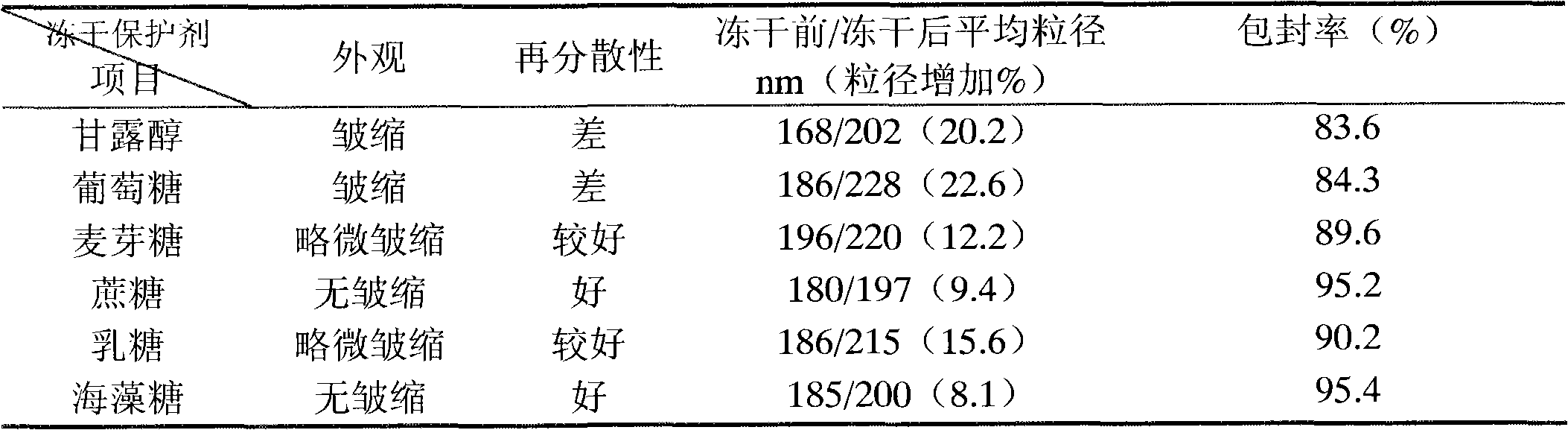
[0040] Embodiment 1: selection of lyoprotectant
[0041] 1. Prescription:
[0042] Alprostadil (PGE 1 ) 5mg
[0043] Refined soybean oil 100g
[0045] Oleic acid 2.4g
[0046] Lyoprotectant 100g
[0047] Appropriate amount of pH adjuster
[0048]
[0049] Add water for injection to 1000ml
[0050] 2. Process:
[0051] (1) Preparation of the water phase: dissolve the lyoprotectant in water, heat to 55-70°C, and set aside;
[0052] (2) Preparation of oil phase: heat refined soybean oil to 55-70°C, add egg yolk lecithin, dissolve oleic acid, add alprostadil, stir to dissolve;
[0053] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 55-70°C, high-speed shear dispersion, shear speed of 8000rpm, and time of 15 minutes to form colostrum
[0054] (4) High-pressure homogenization: Adjust the pH of the colostrum in step (3) to 6.0-7.5...
Embodiment 2
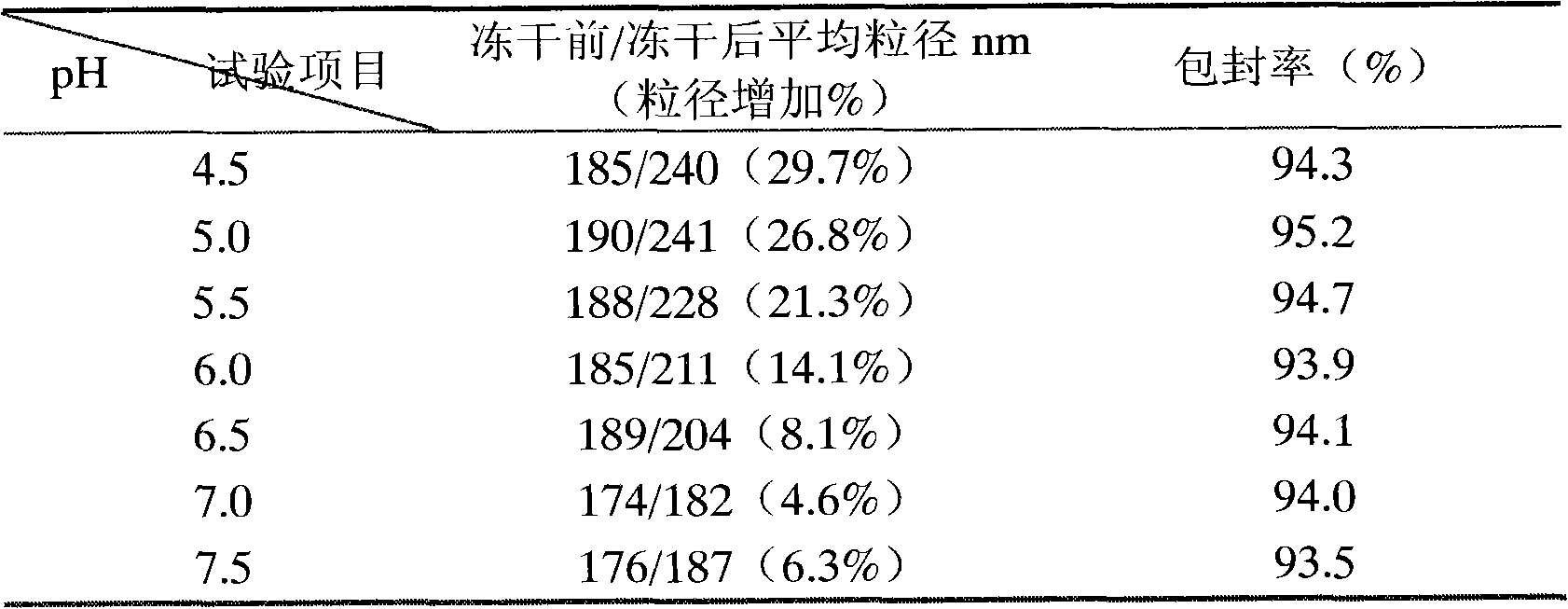
[0076] Embodiment 2: the influence of pH on emulsion particle size and drug stability
[0077] Using sucrose as the freeze-drying protective agent, the effects of different pH values on the particle size of milk particles before and after freeze-drying were investigated, and the average particle size, prostaglandin E 1 and prostaglandin A 1 content as an indicator. The results are shown in Table 2 below.
[0078] 1. Prescription
[0079] Alprostadil (PGE 1 ) 5mg
[0080] Refined soybean oil 100g
[0082] Oleic acid 2.4g
[0083] Sucrose 120g
[0084] Appropriate amount of pH adjuster
[0085]
[0086] Add water for injection to 1000ml
[0087] 2. Process:
[0088] (1) Preparation of the water phase: dissolve the lyoprotectant in water, heat to 55-70°C, and set aside;
[0089] (2) Preparation of oil phase: heat refined soybean oil to 55-70°C, add egg yolk lecithin, dissolve oleic acid, ad...
Embodiment 3
[0103] Embodiment 3: the comparison of freeze-drying process
[0104] Alprostadil submicroemulsion for injection is a very difficult freeze-drying system. Because it also contains liquid oil, phospholipids, oleic acid, etc., the components are relatively complex and the solution is relatively viscous. The requirements for freeze-drying conditions are extremely strict and the control is not good. , it is difficult to freeze-dry and even lead to demulsification and oil separation, and more often lead to the increase of the particle size of the emulsion after reconstitution, which exceeds the limit. Use the conventional freeze-drying method for freeze-drying: pre-freeze at -40°C to -20°C for 2 hours, dry once under reduced pressure at 0-10°C, and then dry at 15-25°C under reduced pressure Secondary drying. As a result, oil precipitation occurred during a drying process, which indicated that this method was not suitable for the freeze-drying of alprostadil submicroemulsion for in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com