Intravaginal drug delivery devices for the delivery of macromolecules and water-soluble drugs
A delivery device and water-soluble technology, applied in drug delivery, drug devices, microorganisms, etc., can solve problems such as inappropriate drug therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0080] The silicone elastomer base MED-6382 is mixed with the cross-linking agent tetrapropyl orthosilicate at a ratio of 40:1. Add tin octoate catalyst (0.5%, w / w), and the mixture is injected into a specially designed vaginal ring mould. The mixture was cured at 80°C for 2 minutes to produce a silicone elastic vaginal ring containing 1 or 3 channels, which had the following dimensions: 7.6 mm cross-sectional diameter, 43.0 mm inner diameter, 58.0 mm outer diameter and 3.0 mm channel insert diameter.
[0081] The DAC 150 FVZ-K Speedmixer mixes the desired content of the drug with the appropriate silicone elastomer at 3000 rpm for 30 seconds to manufacture the drug-loaded silicone rod. The mixture was then injected into a 3mm diameter PVC pipe and cured at room temperature for 24 hours. Cut the PVC pipe to the desired length, and then use a scalpel to carefully remove the PVC shell from the cured silicone elastomer stick. Protocol for in vitro release studies-Examples 1-3
[008...
Embodiment 1
[0084] Those skilled in the art will easily understand that the release rate and release amount demonstrated in the following examples are not restrictive, and can be manipulated to change the water solubility of the insert by, for example, changing the loading amount of the drug substance in the insert The loading and / or type of release enhancer, and / or changing the size of the insert, or a combination of some or all of these parameters, to change the release rate and / or the release amount as required. Example 1 Sustained release of bovine serum albumin from intravaginal drug delivery device
[0085] The silicone elastomer vaginal ring containing a single channel is manufactured according to the general method as described above. According to the general method as described above, it also produced 1.0% w / w BSA, 20.0% w / w water-soluble release enhancer (glycine, glucose, PVP or PEG 3400) and 79.0% w / w LSR-9-9508- 30 Silicone Elastomer (Dow Corning) silicone elastomer insert, whi...
Embodiment 2
25 182.78
28 188.48
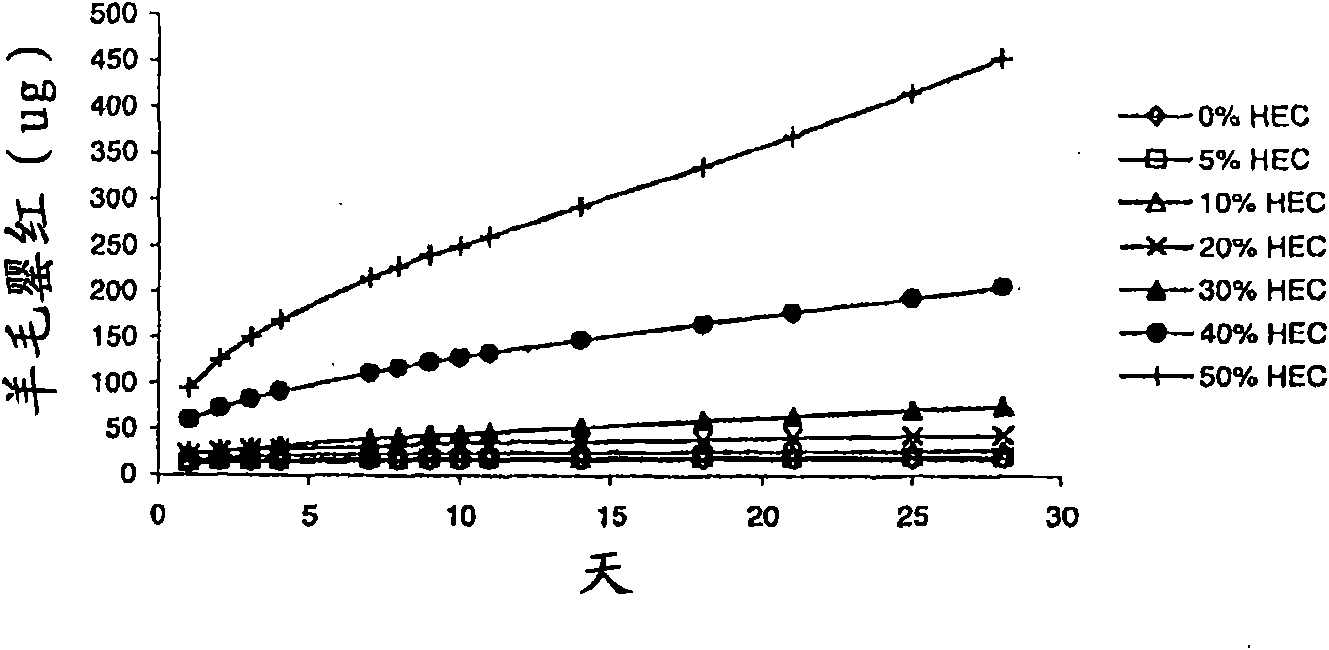
[0089] The drug delivery device was placed in a sample vial with 10ml sterile water; the vial was capped and placed in a 37°C orbital (60rpm) shaking incubator (Unitron HTInfors). Sampling was performed daily for 14 days with completely replaced release medium (sterile water), followed by sampling / replacement twice a week for another 2 weeks. A rod-shaped drug delivery device with a single drug-containing insert therein was used in the dissolution studies conducted in Examples 4-6. If loaded with wool poppy, the rod-shaped insert of BSA or 2F5 is in situ in the body of the ring device, this drug delivery device is an accurate representative of the expected release profile, that is, the drug will only be removed from the drug delivery device. The two open ends are released. Example 4 The effect of the type and concentration of the water-soluble release enhancer on the release of lanolin.
[0090] The silicone elastomer LSR9-9508-30 was provided by NuSil ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com