Nasal pharmaceutical composition of cyclodextrin inclusion cortin and H1 receptor antagonist
A technology of receptor antagonists and corticosteroids, applied in the direction of drug combinations, active ingredients of heterocyclic compounds, non-active ingredients of polymer compounds, etc., can solve the problem of unsatisfactory stability, poor stability of nasal sprays, reduced delivery, etc. problems, to achieve the effect of reducing the possibility of side effects, improving curative effect, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
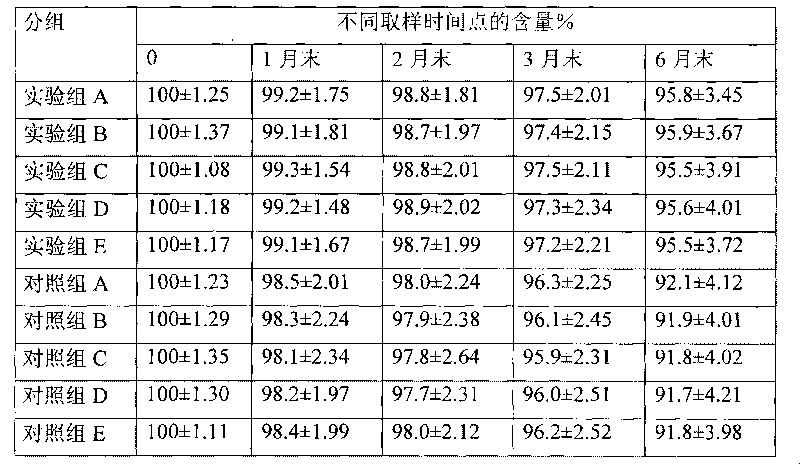
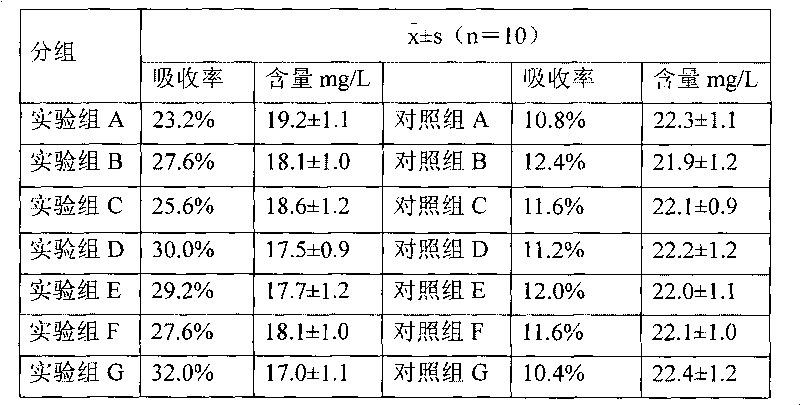
Embodiment 1
[0059] The preparation of embodiment 1 hydrocortisone cyclodextrin inclusion aqueous solution
[0060] Dissolve 2 g of 3-hydroxypropyl β-cyclodextrin in 15 ml of 95% ethanol at room temperature, add 0.2 g of hydrocortisone, stir to dissolve, stir for another 30 minutes, filter through a microporous membrane (220 nm), add 8 ml of injection Water, stirred for 5 minutes, concentrated under reduced pressure, and evaporated to remove ethanol to obtain an aqueous solution of hydrocortisone / 3-hydroxypropyl β-cyclodextrin inclusion complex, which is set aside.
Embodiment 2
[0061] The preparation of embodiment 2 dexamethasone acetate cyclodextrin inclusion aqueous solution
[0062] Dissolve 2g of 2-hydroxypropyl-β-cyclodextrin in 20ml of acetone at 40±2°C, add 0.5g of dexamethasone acetate, stir to dissolve, stir for another 30 minutes, filter through a microporous membrane (220nm), add 3mL of water for injection was stirred for 5 minutes, concentrated under reduced pressure, and 4ml of water for injection was added twice during the concentration process to remove the acetone to obtain dexamethasone acetate / 2-hydroxypropyl-β-cyclodextrin inclusion complex aqueous solution ,spare.
Embodiment 3
[0063] The preparation of embodiment 3 mometasone furoate cyclodextrin inclusion aqueous solution
[0064] Dissolve 7g of 2-hydroxyethyl-β-cyclodextrin in 20ml of absolute ethanol at room temperature, add 0.5g of mometasone furoate, stir to dissolve, stir for another 30 minutes, filter through a microporous membrane (220nm), stir for 5 minutes, concentrate under reduced pressure, add 3.5ml water for injection three times during the concentration process, and evaporate ethanol to obtain mometasone furoate / -hydroxyethyl-β-cyclodextrin inclusion aqueous solution, which is set aside.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com