Fluorine-substituted apatite coating on surface of biologic medical magnesium or alloy thereof and preparation method
A biomedical, apatite technology, applied in the field of medicine, can solve the problems of surrounding tissue inflammation, prolong the crystallization period of bone salt, reduce the stability of new bone tissue, etc., achieve good thermal stability and biological stability, and firm combination , The effect that the degradation cycle can be adjusted
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
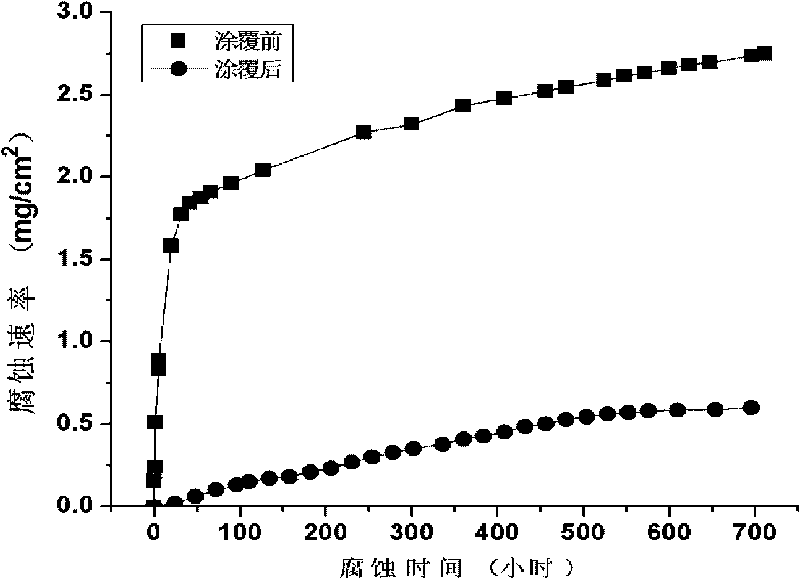
Image
Examples
Embodiment 1
[0026] Step 1, preparing a deposition solution, the composition of 1L of the deposition solution is:
[0027] CaCl 2 0.002mol, NaH 2 PO 4 2- 0.001mol, MgF 2 1×10 -5 mol, the balance is pure water;
[0028] Step 2, after surface cleaning, degreasing and necessary activation process, the biomedical magnesium rare earth alloy stent is soaked in the deposition solution prepared in step 1, and the pH of the deposition solution is adjusted to 7.4;
[0029] The deposition solution was then placed in a cell culture incubator (CO 2 Partial pressure is 5%), the temperature is set to 30 DEG C, after 24 hours, the thickness is 0.1 micron thick coating, the main component of the coating is short rod-shaped carbonate-fluorine mixed substituted apatite, The structural formula is: (Ca, Na 2 ) 10 (PO 4 ) 6-2y (CO 3 ) 3y (OH) 2-z f z , where y=2.9, z=0.1;
[0030] Step 3, annealing the coated bracket at 150° C. for 24 hours can be used as an orthopedic implant material.
Embodiment 2
[0032] Step 1, preparing a deposition solution, the composition of 1L of the deposition solution is:
[0033] 0.1mol Ca(NO 3 )·4H 2 O, 0.05mol NH 4 h 2 PO 4 , 0.01mol NaF, the balance is pure water;
[0034] Step 2, placing the biomedical magnesium-aluminum alloy AZ91 screw in the deposition solution prepared in step 1, adjusting the pH of the deposition solution to 3.0, and the temperature to 55°C, so that the fluorine replaces the apatite coating for spontaneous deposition;
[0035] At the same time, the structure of the coating is controlled by electrochemical assisted deposition. The specific parameters are: the inert electrode silver electrode is used as the anode, and the AZ91 magnesium aluminum alloy is used as the cathode for electroplating, and the current density is controlled at 20mA / cm 2 , the entire deposition process is carried out under the protection of nitrogen or argon, and after 2 hours, acicular fluorine-substituted hydroxyapatite coating with a thickn...
Embodiment 3
[0039] Step 1, preparing a deposition solution, the composition of 1L of the deposition solution is:
[0040] Ca(NO 3 ) 2 1mol, NaHPO 40.5mol, SnF 0.1mol, anti-precipitation agent NaSiO 3 12H 2 O 0.01mol, the balance is pure water;
[0041] Step 2, adjust the pH of the deposition solution to 5.0, and the temperature to 90°C; use the biomedical Mg-1%Zn-%1Ca ternary magnesium alloy as the anode, and use the inert electrode as the cathode to carry out anodic oxidation. The specific reaction parameters are: anode voltage 95V, the current density is 160mA / cm 2 , the treatment time was 600 minutes; as a result, a coating with a thickness of 2 mm was obtained, and the main component of the coating was acicular fluorine mixed substituted apatite, (Ca, Mg, Na 2 ) 10 (PO 4 ) 6 (OH) 2-x f x , where x=1.9;
[0042] In step three, the ternary magnesium alloy covered with the coating obtained in step two is annealed at 400 ° C for 0.5 hours, which can be used as a medical implan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com