Xylanase XYNA4 with wide pH applicability and gene and application thereof
A xylanase and gene technology, applied in the field of genetic engineering, can solve the problems of cloning and expression without related reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Isolation and purification of Alicyclobacillus hesperidum A4 (CGMCC No.3147) and its enzyme-producing properties
[0047] Soil samples collected from high hot springs in Yunnan were transferred to enrichment medium (yeast extract 1.0g, tryptone 2.0g adjusted to pH 2.0 with hydrochloric acid), cultured at 60°C for 72 hours, diluted and plated to the separation medium ( Yeast extract 1.0g, tryptone 2.0g, oat xylan 5.0g, agar 30, Congo red 0.5g, adjust the pH to 3.0 with hydrochloric acid) After culturing at 60°C for 72 hours, pick according to the presence and size of the transparent circle An enzyme-producing strain, numbered A4, was identified as Alicyclobacillus hesperidum A4 based on 16SRNA and strain characteristics. Alicyclobacillus hesperidum A4 was then transferred to the growth medium (1.0 g of yeast extract, 2.0 g of tryptone and adjusted to pH 3.0 with hydrochloric acid), and its optimum growth temperature and pH value were tested. The results showed...
Embodiment 2
[0048] Example 2 Cloning of Alicyclobacillus hesperidum A4 (CGMCC No.3147) Xylanase Encoding Gene XYNA4
[0049] Extract Alicyclobacillus hesperidum A4 (CGMCC No.3147) genomic DNA:
[0050] Centrifuge the cultured bacteria for 2 days, add 1mL lysozyme, treat at 37°C for 60min, then add lysate, lyse in a water bath at 65°C for 30min, mix every 10min, and centrifuge at 10,000rpm at 4°C for 5min. The supernatant was extracted in phenol / chloroform to remove impurity proteins, and then an equal volume of isopropanol was added to the supernatant. After standing at room temperature for 5 minutes, centrifuge at 10,000 rpm for 10 minutes at 4°C. The supernatant was discarded, the precipitate was washed twice with 70% ethanol, dried in vacuum, dissolved by adding an appropriate amount of TE, and stored at -20°C for later use.
[0051] The degenerate primers XYN10F and XYN10R were designed and synthesized according to the conserved (YDWDV and HGIGM) sequences of the tenth family xylanas...
Embodiment 3
[0058] Example 3 Preparation of recombinant xylanase.
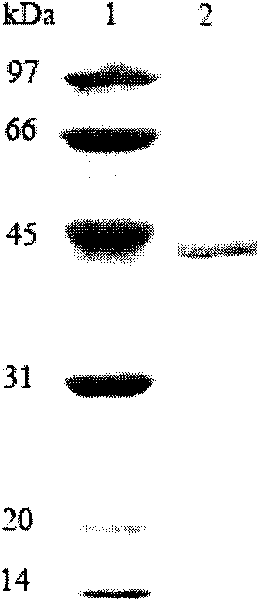
[0059] The expression vector PET-22B(+) was double digested (EcoRI+Hind III), and the gene XYNA4 encoding xylanase was double digested (EcoRI+Hind III) to cut out the gene encoding mature xylanase The fragment was connected with the expression vector pPET-22B(+), and the recombinant plasmid PET-22b(+)-XYNA4 containing Alicyclobacillus hesperidum A4 (CGMCC No.3147) xylanase gene XYNA4 was obtained and transformed into Escherichia coli BL21(DE3), and obtained Recombinant E. coli strain BL21(DE3) / XYNA4.
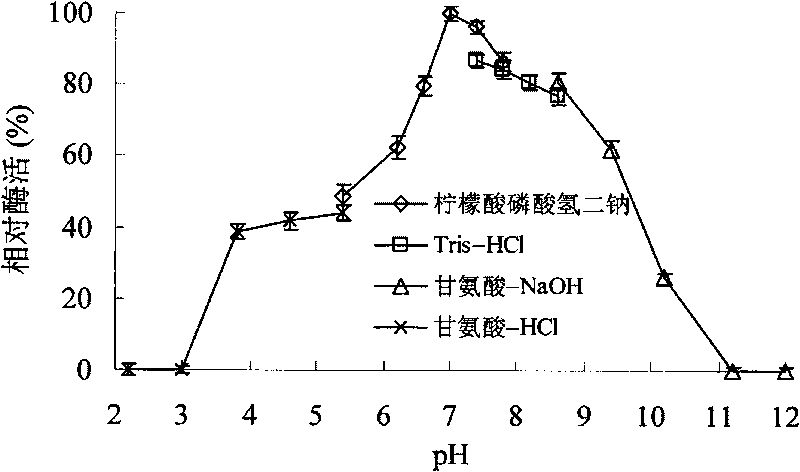
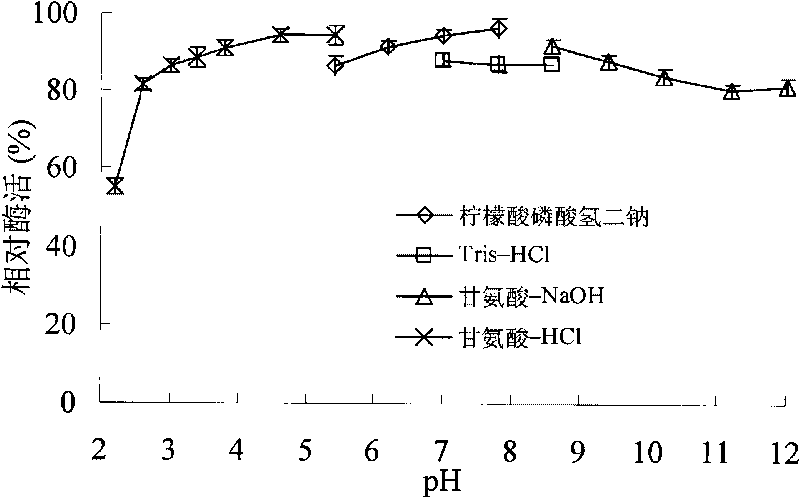
[0060] Take the BL21(DE3) strain containing the recombinant plasmid, inoculate it in 200mL LB culture medium (1000ml Erlenmeyer flask), shake it at 250rpm at 37°C for 3-4h, and add IPTG for induction. After shaking culture at 250 rpm at 30°C for 10-12 hours, the supernatant was collected by centrifugation. Determination of xylanase activity. The expression level of recombinant xylanase was 0.38U / mL. After the expressed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com