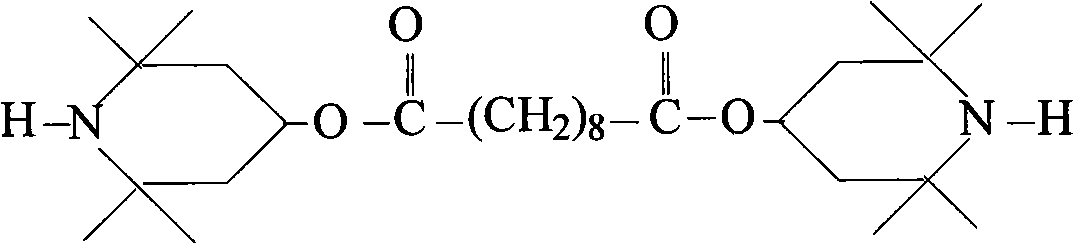Light stabilizer bis(2,2,6,6-tetramethyl-4-piperidinyl)sebacate and preparation method thereof
A technology of dimethyl sebacate and sebacic acid is applied in the field of light stabilizer sebacate diester and its production, which can solve the problems of large pollution, high risk, and easily corroded equipment, and achieve simplified separation Purification process, short reaction time, small effect of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a 250mL four-necked flask equipped with a thermometer, add xylene 40g, dimethyl sebacate 23.0g (0.1mol), 2,2,6,6-tetramethyl-4-hydroxypiperidine 33.03g ( 0.21mol), catalyzer 1g, this catalyzer is dibutyl tin oxide, loads on condenser, starts agitator, when heating up to 110-130 ℃, the methyl alcohol that generates constantly is steamed out in the reaction process, reaction time 6h (using liquid phase Chromatographic monitoring of the reaction). After the reaction, filter and wash with water, collect the organic phase, remove water, decolorize and crystallize the organic phase, and filter to obtain white crystalline powder sebacic acid bis(2,2,6,6-tetramethyl-4-piperidine Base) ester, the product yield is 97.85%.
Embodiment 2
[0019] In a 250mL four-necked flask equipped with a thermometer, add xylene 40g, dimethyl sebacate 23.0g (0.1mol), 2,2,6,6-tetramethyl-4-hydroxypiperidine 33.03g ( 0.21mol), catalyzer 1.2g, this catalyzer is dibutyltin oxide, puts on condenser, starts agitator, when heating up to 110-130 ℃, the methyl alcohol that generates constantly is steamed out in the reaction process, reaction time 6h (with liquid The reaction was monitored by phase chromatography). After the reaction, filter and wash with water, collect the organic phase, remove water, decolorize and crystallize the organic phase, and filter to obtain white crystalline powder sebacic acid bis(2,2,6,6-tetramethyl-4-piperidine Base) ester, the product yield is 98.06%.
Embodiment 3
[0021] In a 250mL four-necked flask equipped with a thermometer, add xylene 40g, dimethyl sebacate 23.0g (0.1mol), 2,2,6,6-tetramethyl-4-hydroxypiperidine 33.82g ( 0.215mol), catalyzer 1.0g, this catalyzer is dibutyl tin oxide, puts on condenser, starts agitator, when heating up to 110-130 ℃, the methyl alcohol that generates constantly is distilled in the reaction process, reaction time 6h (with liquid The reaction was monitored by phase chromatography). After the reaction, filter and wash with water, collect the organic phase, remove water, decolorize and crystallize the organic phase, and filter to obtain white crystalline powder sebacic acid bis(2,2,6,6-tetramethyl-4-piperidine Base) ester, the product yield is 98.05%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com