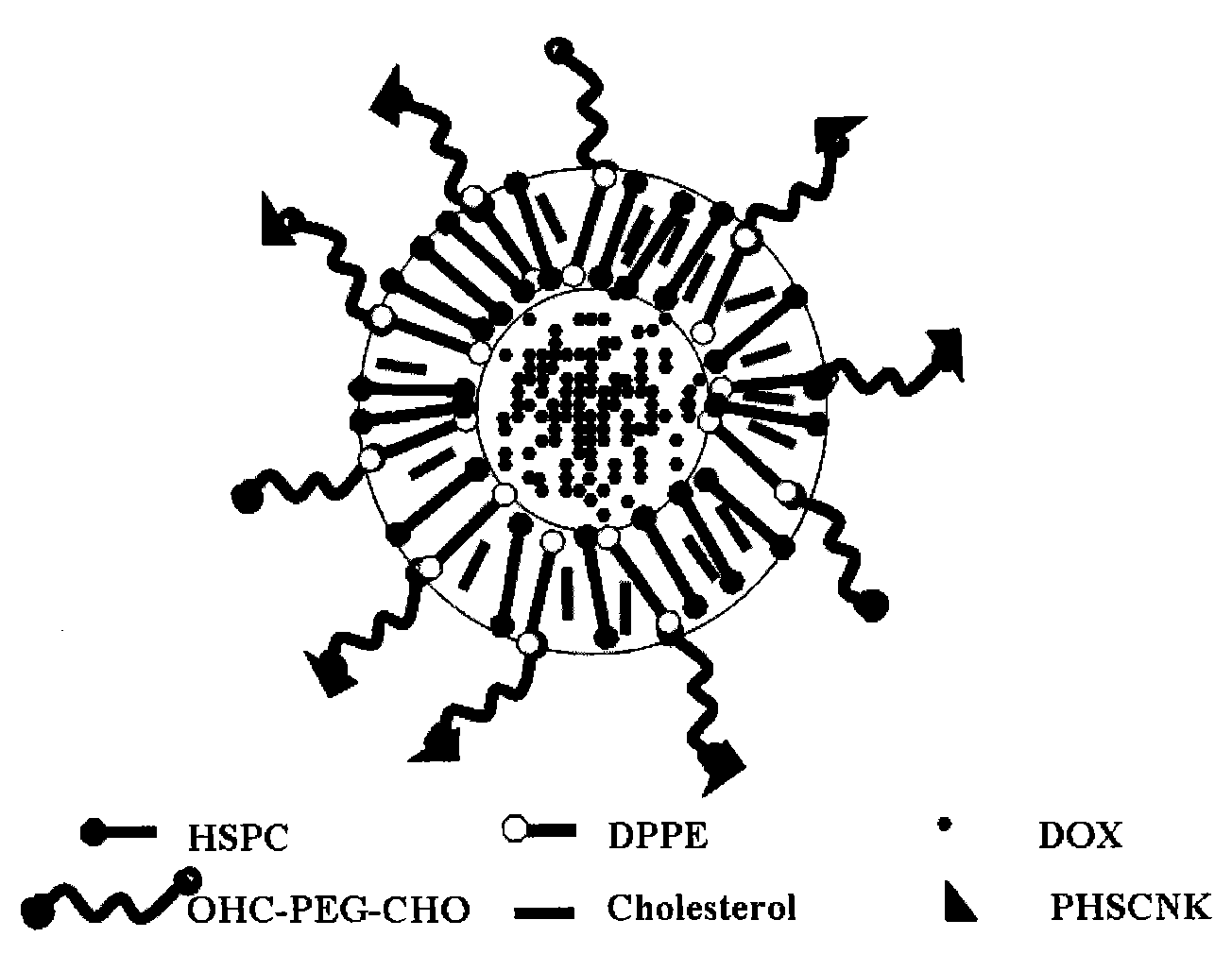
Tumor dual target liposome mediated by integrin and preparation method thereof
A technology of liposome and liposome composition, which is applied in the directions of liposome delivery, anti-tumor drugs, medical preparations of inactive ingredients, etc. , the effect of inhibiting tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of the long circulation liposome of the PHSCNK modification of encapsulating doxorubicin
[0044] Take hydrogenated soybean lecithin, cholesterol, dipalmitoylphosphatidylethanolamine, OHC-PEG2000-CHO (molar ratio is 15:10:5:10), put in a round bottom flask, add appropriate amount of chloroform:methanol (10:1), After ultrasonic dissolution, heat in a water bath and vacuum rotary evaporation to remove the organic solvent to form a uniform transparent film. Draw an appropriate amount of ammonium sulfate solution (123mM, pH 5.4) into a round-bottomed flask, vortex and oscillate to completely shed the lipid film, and sonicate in a water bath until light blue opalescence appears. Pass the prepared blank liposomes through a Sephadex G50 column, elute with phosphate buffered saline (PBS, pH 7.4), collect the liposome fraction, and preheat the collected blank liposomes in a water bath at 75°C. Add doxorubicin stock solution and incubate for 10 minu...
Embodiment 2
[0048] Example 2. In vitro cell-targeting evaluation of PHSCNK-modified long-circulating liposomes loaded with doxorubicin
[0049] The uptake of doxorubicin by cells and the distribution of doxorubicin in cells were investigated by laser confocal experiment. Human umbilical vein endothelial cells or melanoma cells were placed in CO with PL-DOX and PHSCNK-PL-DOX, respectively. 2 Incubate in the incubator for 2 hours, rinse with cold PBS buffer for 3 times in sequence, fix the tissue cells with fixative solution, then stain the nucleus with Hoechst 33258 for 10 minutes, rinse with PBS for 3 times, seal with PBS, refrigerate in the dark, and use laser confocal Microscopic determination. The experimental results showed that PHSCNK-modified doxorubicin long-circulating liposomes could significantly increase the drug uptake by endothelial cells and tumor cells.
Embodiment 3
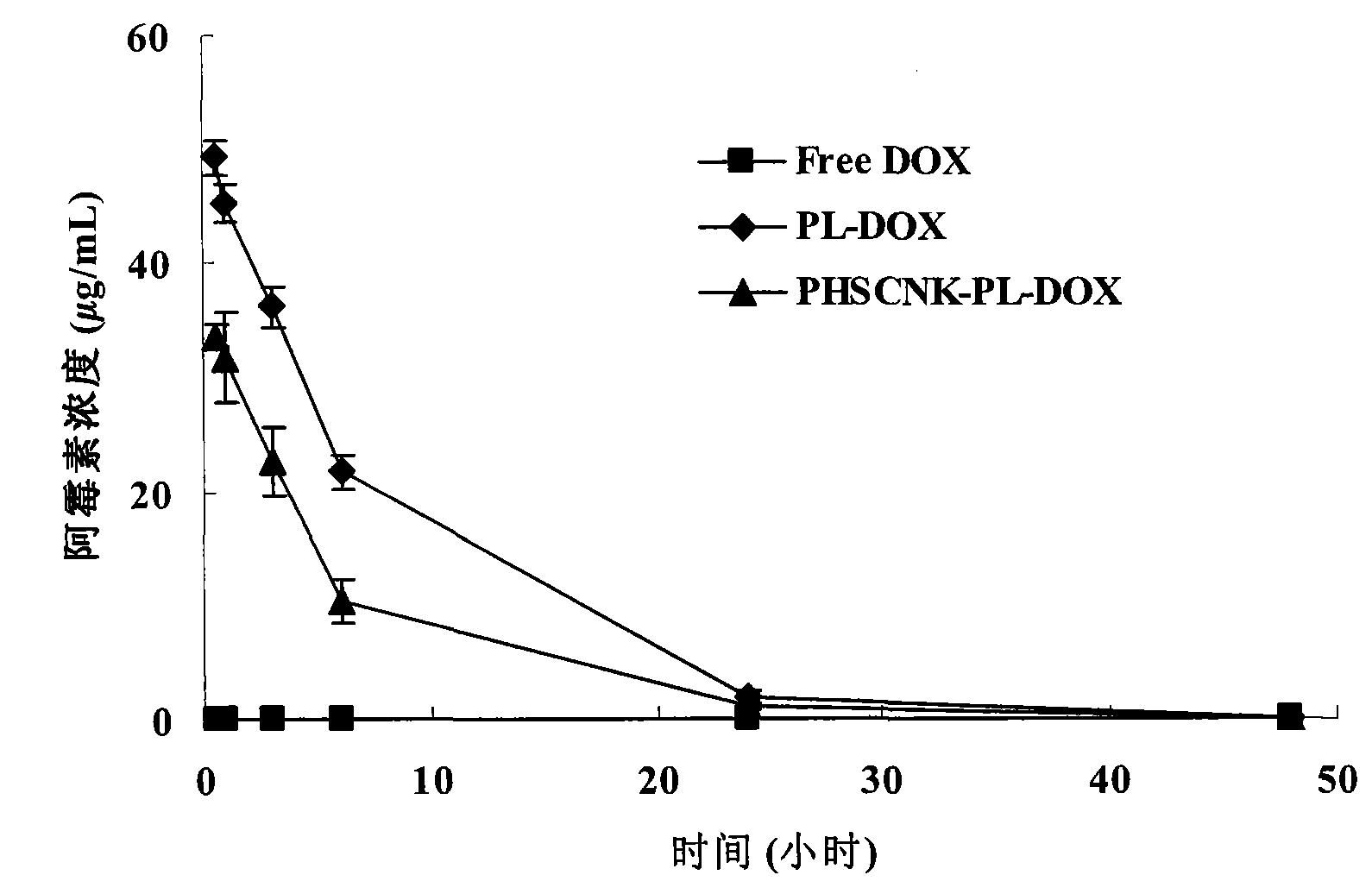
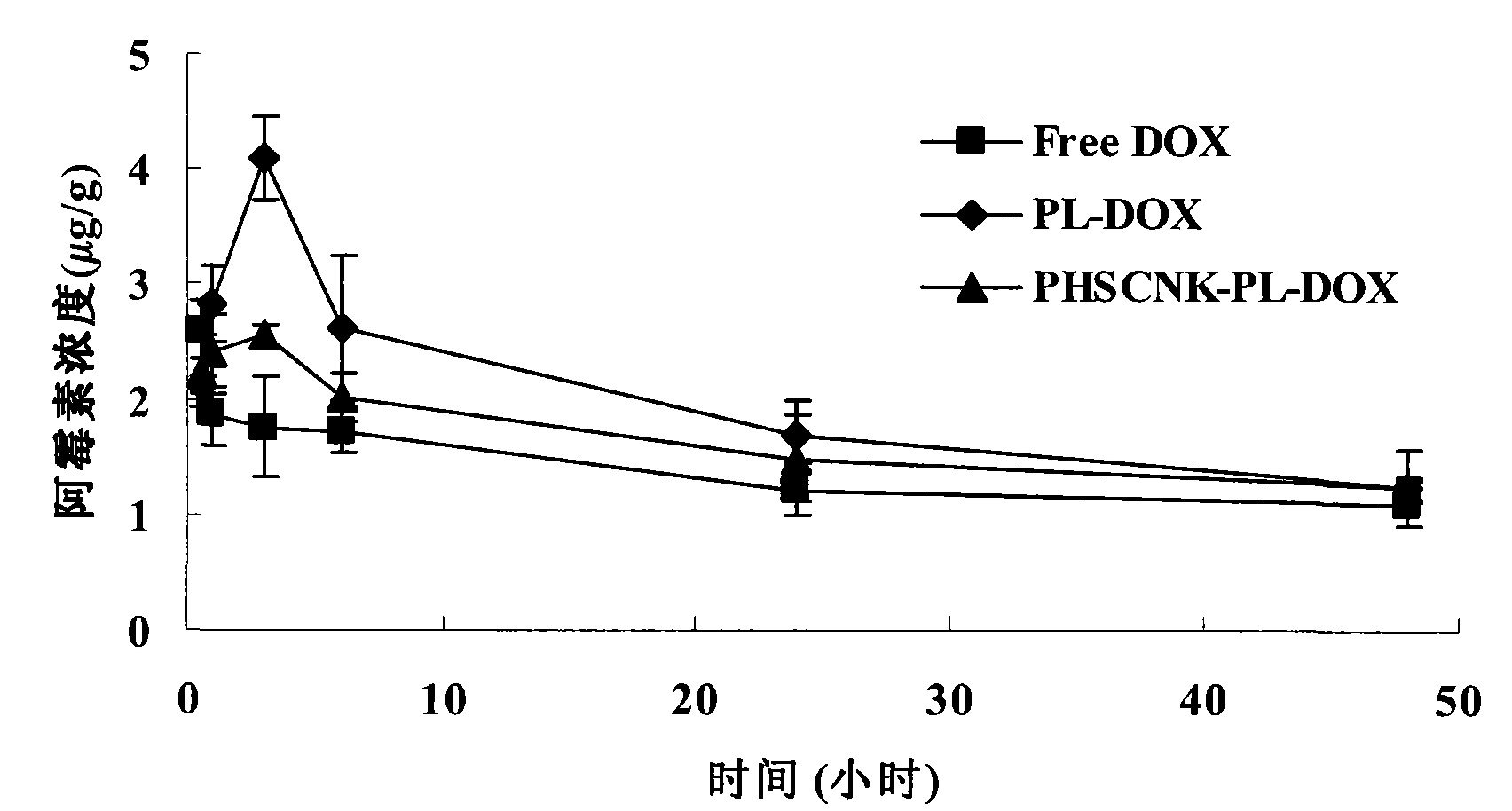
[0050] Example 3, Pharmacokinetics of PHSCNK-modified long-circulating liposomes loaded with doxorubicin and distribution in tumor tissues
[0051] Since the present invention combines the long-circulation liposome technology and the method of promoting intracellular transport to improve the curative effect of anti-cancer drugs, it is required to possess the characteristics of long-circulation liposomes, that is, to delay the clearance of drugs in plasma and the delivery of drugs to tumor tissues. and transport in tumor cells. In this example, C57BL / 6 mice inoculated with melanoma B1610 were used as an animal model, and the experimental results after tail vein injection of doxorubicin preparation.
[0052] Reference preparations: doxorubicin long-circulation liposome, free doxorubicin solution.
[0053] Test preparation: PHSCNK-modified long-circulating liposomes loaded with doxorubicin.
[0054] Experimental animals: C57BL / 6 mice inoculated with mouse-derived melanoma B16F1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com