Levetiracetam slow release pellet capsule preparation
A technology of sustained-release pellets and capsules, which is applied in the field of levetiracetam sustained-release pellets and capsule preparations and its preparation, can solve the problems of large individual differences in gastric emptying, achieve improved bioavailability, small individual differences, good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
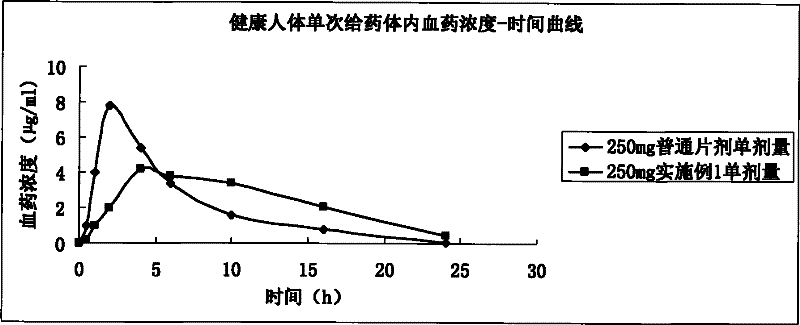
Image
Examples
Embodiment 1
[0031] Levetiracetam Extended Release Pellets Capsules Prescription: (Quality)
[0032] Levetiracetam 62%
[0033] Blank ball core (diameter 350 micron-450 micron) 18%
[0034] Hypromellose E5 6.5%
[0035] Ethyl cellulose aqueous dispersion E-7-19040 (solid content) 12%
[0036] Talc 1%
[0037] Colloidal silicon dioxide 0.5%
[0038] Preparation method of levetiracetam sustained-release pellets and capsules:
[0039] Measured according to the prescription, levetiracetam was pulverized through a 100-mesh sieve, and hydroxypropylmethylcellulose E5 was prepared into a 5% solution with purified water to be used as a binder. Add the blank ball core (diameter 350 micron-450 micron) in the sugar-coating machine (diameter 60 centimeters), adjust the rotating speed 20-25 rev / min, elevation angle 10-20 °, make the blank ball core be in motion, then put 5 % hydroxypropylmethylcellulose E5 aqueous solution binder is sprayed in through a spray gun (atomization pressure 0.02 MPa, at...
Embodiment 2
[0051] Levetiracetam Extended Release Pellets Capsules Prescription: (Quality)
[0052] Levetiracetam 62%
[0053] Blank ball core (diameter 350 micron-450 micron) 18%
[0054] Hypromellose E5 6.5%
[0055] EUDRAGIT NE 30D (solid content) 8%
[0056] Talc 5%
[0057] Colloidal silicon dioxide 0.5%
[0058] Preparation method of levetiracetam sustained-release pellets and capsules:
[0059] Measured according to the prescription, levetiracetam was pulverized through a 100-mesh sieve, and hydroxypropylmethylcellulose E5 was prepared into a 5% solution with purified water to be used as a binder. Blank ball core (diameter 350 micron-450 micron) is added in the sugar-coating machine (diameter 60 centimeters), adjust rotating speed 20-25 rev / min, elevation angle 10-20 °, make blank ball core be in motion state, then put 5 % hydroxypropylmethylcellulose E5 aqueous solution binder is sprayed in through a spray gun (atomization pressure 0.02 MPa, atomization distance 10-20 cm) to...
Embodiment 3
[0064] Levetiracetam Extended Release Pellets Capsules Prescription: (Quality)
[0065] Levetiracetam 60%
[0066] Blank ball core (diameter 350 micron-450 micron) 18%
[0067] Povidone K30 8.5%
[0068] Ethyl cellulose aqueous dispersion E-7-19040 (solid content) 12%
[0069] Talc 1%
[0070] Colloidal silicon dioxide 0.5%
[0071] Preparation method of levetiracetam sustained-release pellets and capsules:
[0072] Measured according to the prescription, the levetiracetam is pulverized through a 100-mesh sieve, and the povidone K30 is prepared into a 5% solution with purified water to be used as a binder. Add the blank ball core (diameter 350 micron-450 micron) in the sugar-coating machine (diameter 60 centimeters), adjust the rotating speed 20-25 rev / min, elevation angle 10-20 °, make the blank ball core be in motion, then put 5 100% povidone K30 aqueous solution binder is sprayed in through a spray gun (atomization pressure 0.02 MPa, atomization distance 10-20 cm), an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com