One pot method for preparing aryl-alpha-keto ester based on arylethyl ketone
A technology of aryl ethyl ketone and keto ester, which is applied in the preparation field of organic chemical technology, can solve the problems of high synthesis cost, difficulty in obtaining aryl acetate, difficult reaction operation, etc., and achieve low synthesis cost and high source of raw materials Convenience and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
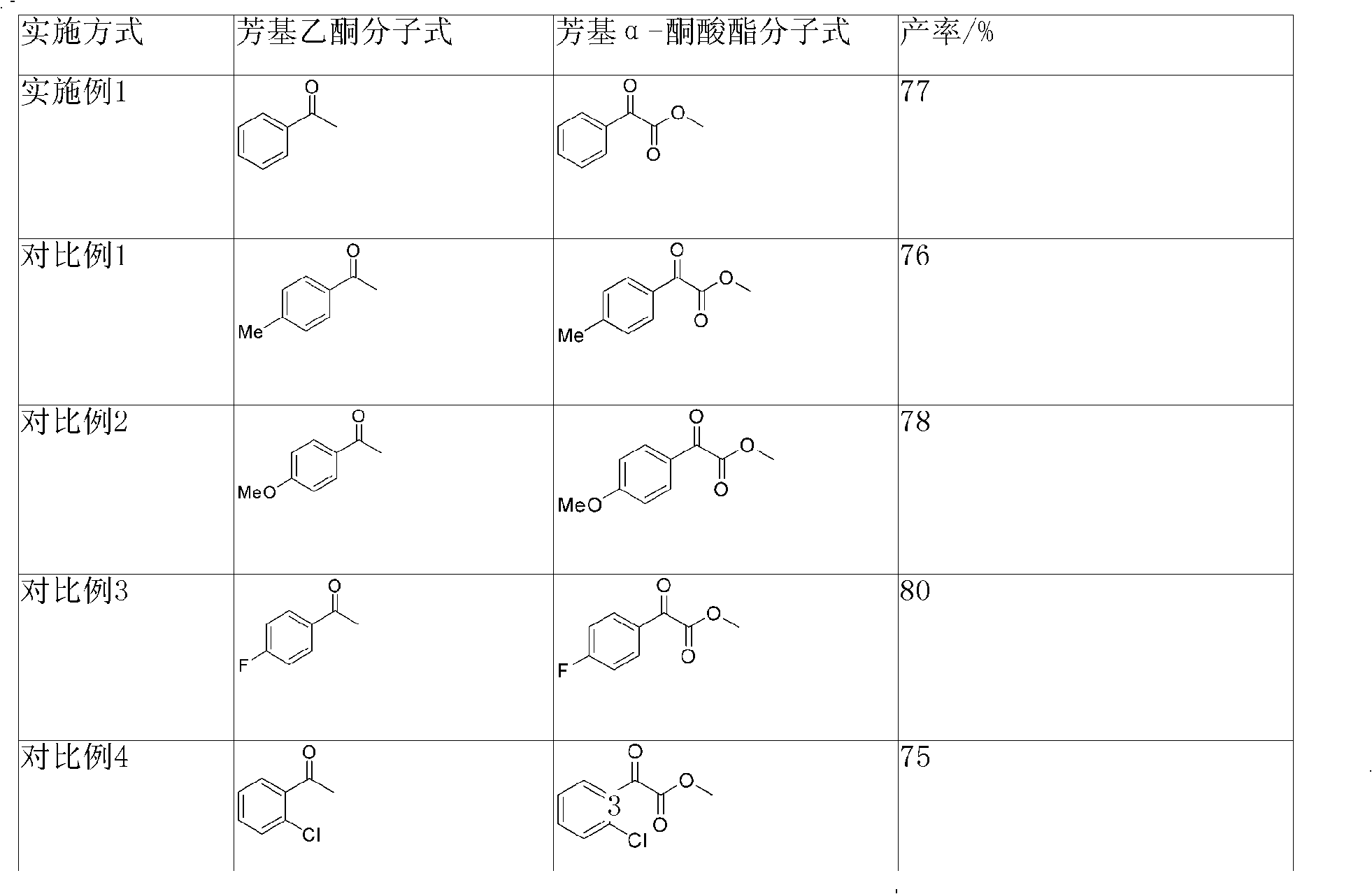
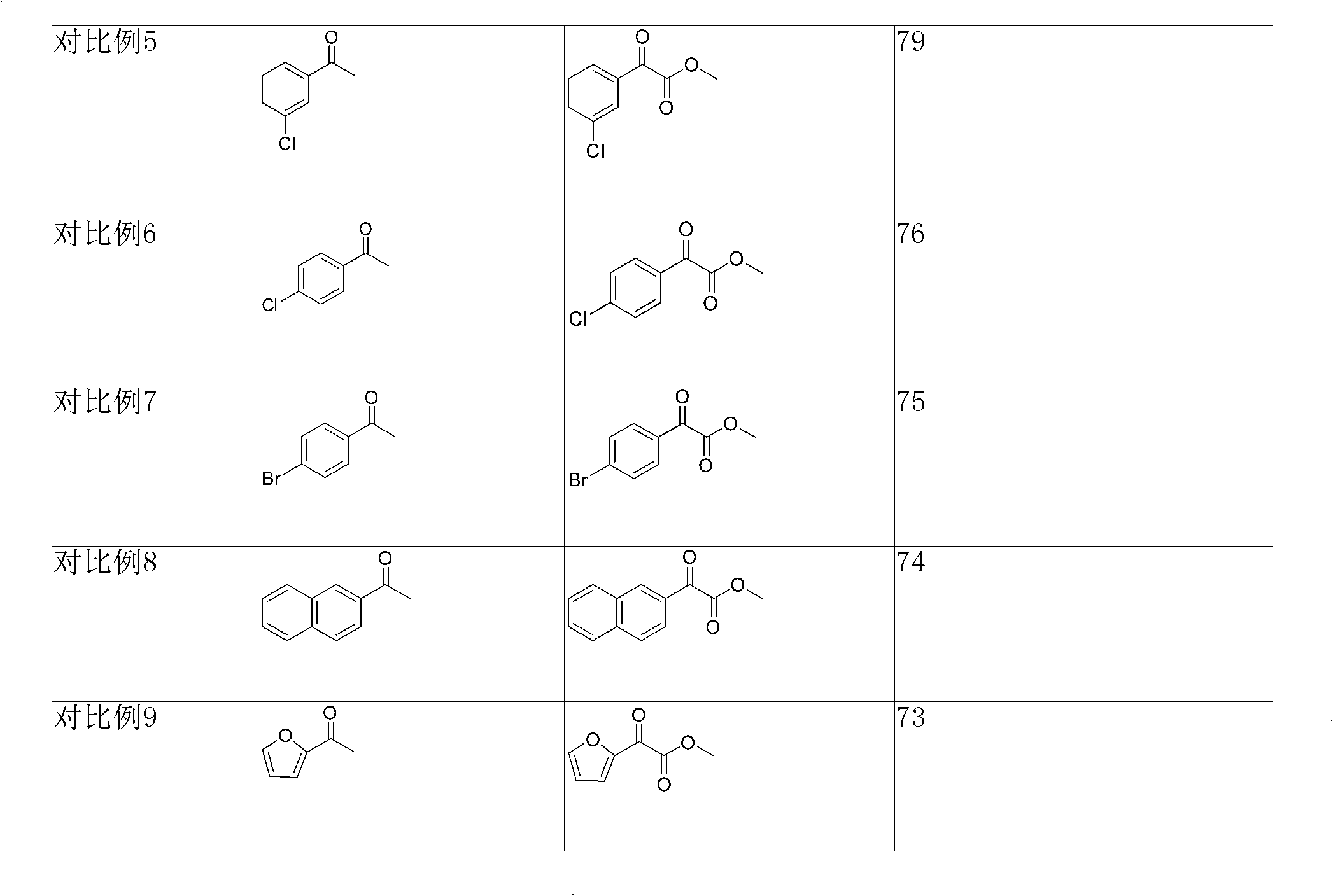
Examples
Embodiment 1
[0024] Such as figure 1 Shown, the present embodiment preparation method comprises the following steps:
[0025] Under nitrogen protection, acetophenone (0.48g, 4.0mmol), selenium dioxide (0.66g, 6.0mmol), pyridine (2.0mL, 25mmol) were added to a 25mL two-necked flask, and the oil bath was slowly heated to 100°C , after reflux stirring for 12h, TLC detects that raw material point 1 disappears;
[0026] In an ice-water bath environment, add 0.24g molecular sieves and methanol (2.9mL, 72mmol) to the two-necked flask, after proper stirring, slowly add thionyl chloride (1.5mL, 20mmol) dropwise, and remove the ice after about 1 hour. bath, stirring at room temperature for 16 hours;
[0027] Add 70-72% perchloric acid (1.6mL, 20mmol), acetonitrile (32mL) and deionized water (3.2mL) and stir the reaction for 0.5h, then gradually add saturated sodium bicarbonate to remove excess acid, and wait for the two-necked flask to Bubbles are generated again, stop stirring, filter to remove ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com