Formation method of lithium ion secondary battery
A secondary battery and formation method technology, applied in secondary batteries, secondary battery charging/discharging, secondary battery repair/maintenance, etc., can solve the initial capacity, cycle performance and low-temperature rate discharge performance of lithium-ion secondary batteries To improve the ionic conductivity and low-temperature applicability and ensure the electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
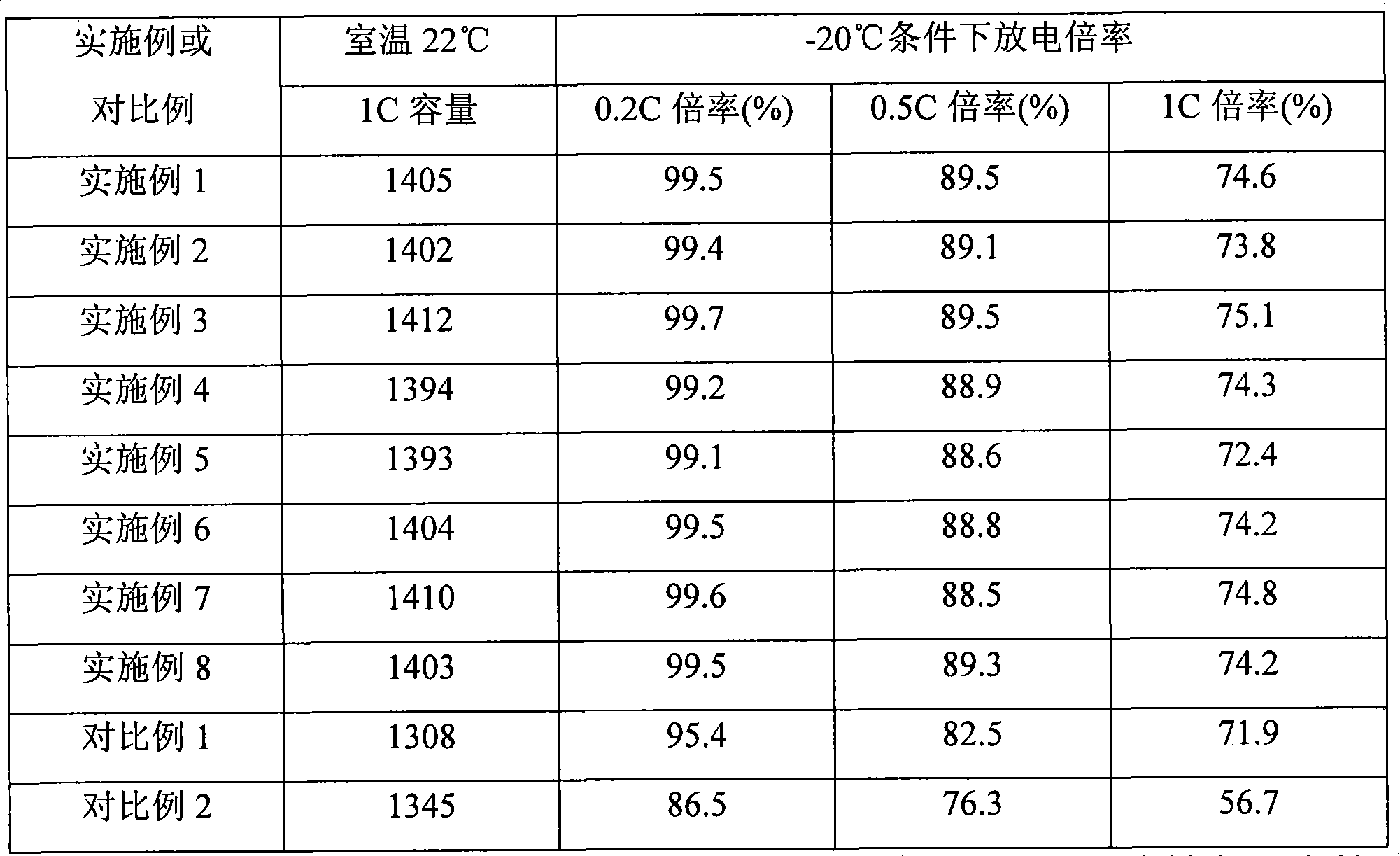
Examples
Embodiment 1
[0026] This embodiment is used to illustrate the preparation of the lithium ion secondary battery provided by the present invention
[0027] 1. Preparation of positive electrode
[0028] 60 grams of polyvinylidene fluoride (PVDF) was dissolved in 770 grams of N-methylpyrrolidone (NMP) solvent to prepare a binder solution, and then 2000 grams of positive electrode active material lithium cobaltate (LiCoO 2 ) and 40 grams of acetylene black powder are added to the above solution, then 200 grams of N-methylpyrrolidone (NMP) is added, fully stirred and mixed to obtain a positive electrode slurry; the positive electrode slurry is evenly coated on the Both sides of the aluminum foil were heated and dried under vacuum at 125°C for 2 hours, rolled, and cut into pieces to obtain a positive electrode of 750 mm (length) × 55.5 mm (width) × 183 microns (thickness). Each positive electrode contained 10 grams of positive active material LiCoO 2 .
[0029] 2. Preparation of negative elect...
Embodiment 2
[0039] This embodiment is used to illustrate the preparation of the lithium ion secondary battery provided by the present invention
[0040] A lithium-ion secondary battery was prepared according to the method of Example 1, except that in the step of preparing the electrolyte, the first electrolyte did not contain the additive VC. Lithium-ion secondary battery A2 was obtained after the formation.
Embodiment 3
[0042] This embodiment is used to illustrate the preparation of the lithium ion secondary battery provided by the present invention
[0043] Lithium-ion secondary battery is prepared according to the method of Example 1, the difference is that in the preparation step of electrolyte, ethylene carbonate (EC), diethyl sulfite (DES) and dimethyl carbonate (DMC) are prepared according to The mass ratio is 1:3:1 and the LiPF 6 Dissolved in the above mixed solvent to obtain the first electrolyte, in which LiPF 6 The content is 15.1% by weight of the total amount of the electrolyte, and 0.5% by weight of the additive vinylene carbonate (VC) is added to the total amount of the first electrolyte.
[0044] Mix γ-butyrolactone (GBL) and ethyl methyl carbonate (EMC) according to the mass ratio of 1:2, and LiPF 6 Dissolved in the above-mentioned mixed solvent to obtain the second electrolyte, LiPF in the electrolyte 6 The content of is 10.2% by weight of the total electrolyte solution. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com