Method for measuring content of ginsenoside in Aidi preparation
A technology of ginsenosides and determination methods, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of not being able to effectively control the quality of Aidi injection preparations, absorption peaks of impurities, and affecting the clinical efficacy of preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Content determination:
[0084] Ginsenoside Re
[0085] Measure according to Chinese Pharmacopoeia 2005 edition an appendix VI D high performance liquid chromatography:
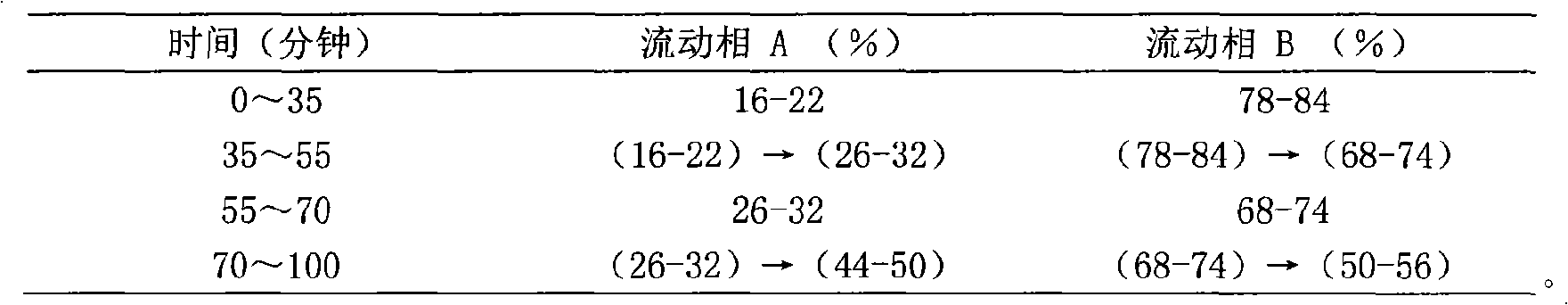
[0086] Chromatographic conditions and system suitability test use octadecylsilane bonded silica gel as filler; use acetonitrile as mobile phase A and water as mobile phase B, perform gradient elution in the following table, and the detection wavelength is 203nm. The number of theoretical plates calculated based on the peak of ginsenoside Rg1 should not be less than 6000;
[0087]
[0088] Preparation of reference substance solution: Accurately weigh ginsenoside Rg1 reference substance, ginsenoside Re reference substance, and ginsenoside Rb1 reference substance, add methanol to make a mixed solution containing 0.1 mg per 1 mL, and shake well to obtain the reference substance solution;
[0089] Preparation of the test solution: Accurately measure 25ml of this preparation, extract 4 times with water-...
Embodiment 2
[0092] Ginsenoside Re
[0093] Measure according to Chinese Pharmacopoeia 2005 edition an appendix VI D high performance liquid chromatography:
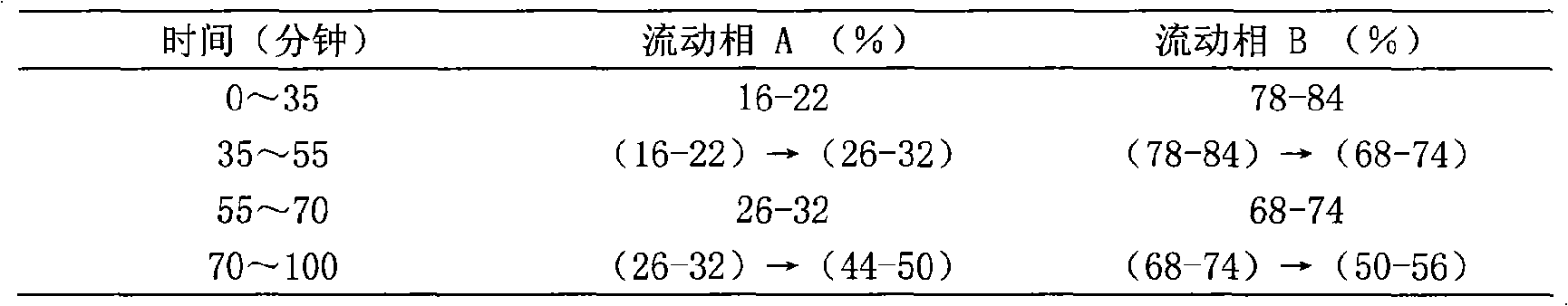
[0094] Chromatographic conditions and system suitability test use octadecylsilane bonded silica gel as filler; use acetonitrile as mobile phase A and water as mobile phase B, perform gradient elution in the following table, and the detection wavelength is 203nm. The number of theoretical plates calculated based on the peak of ginsenoside Rg1 should not be less than 6000;
[0095]
[0096] Preparation of reference substance solution: Accurately weigh ginsenoside Rg1 reference substance, ginsenoside Re reference substance, and ginsenoside Rb1 reference substance, add methanol to make a mixed solution containing 0.2 mg per 1 mL, and shake well to obtain the reference substance solution;
[0097] Preparation of the test solution: Accurately measure 50ml of this preparation, extract 5 times with water-saturated n-butanol, respectively...
Embodiment 3
[0100] Measure according to Chinese Pharmacopoeia 2005 edition an appendix VI D high performance liquid chromatography:
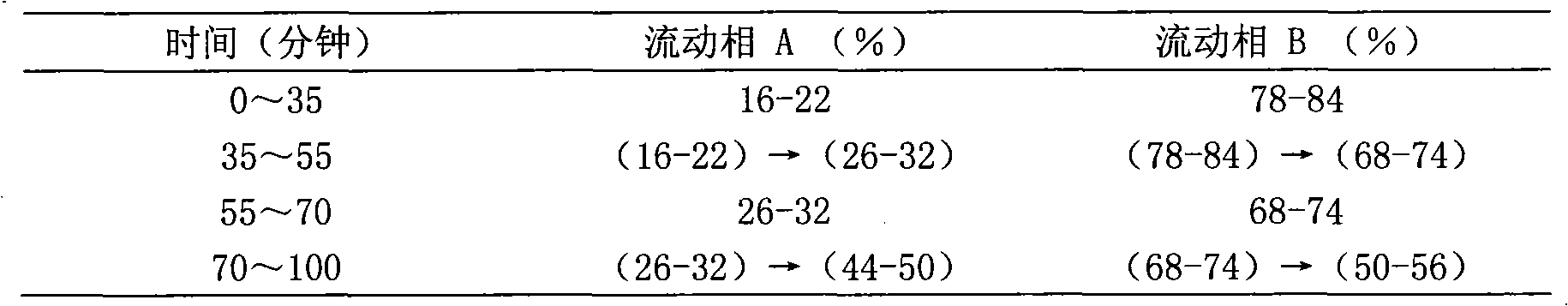
[0101] Chromatographic conditions and system suitability test use octadecylsilane bonded silica gel as filler; use acetonitrile as mobile phase A and water as mobile phase B, perform gradient elution in the following table, and the detection wavelength is 203nm. The number of theoretical plates calculated based on the peak of ginsenoside Rg1 should not be less than 6000;
[0102]
[0103] Preparation of reference substance solution: Accurately weigh ginsenoside Rg1 reference substance, ginsenoside Re reference substance, and ginsenoside Rb1 reference substance, add methanol to make a mixed solution containing 0.4 mg per 1 mL, and shake well to obtain the reference substance solution;
[0104] Preparation of the test solution: Accurately measure 100ml of this preparation, extract 6 times with water-saturated n-butanol, respectively 25mL, 25mL, 20mL, 20mL,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com